DaoxinDai*,XiaoyuMa,XiaojuanYanandXijunBao
FosunCosmetics(Shanghai)Bio-TechnologyCo.,Ltd.,333GuiPingRoad,Shanghai200233,China
*Correspondence:daidaoxin2022@163.com
Abstract: Applying natural mineral water to skin care is a popular tendency and many cosmetics products based on thermal spring water have been developed.The special location and environmental conditions (DSW)with unique ion compositional concentrations, which bring comprehensive positive effects on skin health. This article reviews two potential action modes of DSW, and the biological function of DSW and its related complex in dermatology and skin care. Previous studies have proved the functions of skin moisturization, anti-inflammation, skin barrier repair, and anti-pollution. Especially, the anti-aging effect of DSW and related complexes can act in different ways: keratinocyte rejuvenation,photo-protection,and cellular energy elevation. Additionally, the issues that need further investigation are also discussed.We hope that this review will help to improve the understanding of DSW and its related complex, and further contribute to product development in the skincare industry.
Keywords: Dead Sea; natural mineral water; skin health; cosmetics;molecular mechanism
1. Introduction
The Dead Sea, located on the border of Israel, Palestine, and Jordan, is the lowest point on the continent and one of the three most saline lakes in the world, with a salinity of about 300‰. The extreme environmental conditions have shaped the Dead Sea into a forbidden area for higher plants and animals.Only a few salt-tolerant plants and microorganisms cansurviveontheshoreoraroundthelake. Nevertheless,theskincarebenefitsofDead Sea water (DSW) have been known since biblical times [1], mainly due to its unique ionic concentration and composition.
Compared to other natural waters,suchasordinaryseawaterandhotsprings,DSW has a very high ratio Of divalenttomonovalentcation concentrations. Themaindivalent cations are magnesium, calcium, and strontium, and the main monovalent cations are sodium and potassium. Additionally, the highest concentration of anions is not chloride ion,but bromineion,andDSWalsocontainsometracemetalelements, such as zinc and manganese. The elemental composition of DSW is shown in Table 1. Numerous experimental and cohort studies have proved the therapeutic properties of DSWindermatologicalconditions.TherapeuticbathingintheDeadSeacansignificantly improve skin dryness, peeling, itching, and pain, and alleviate the related inflammation [2]. Allthesearethecommonsymptomscausedbychronicskindiseases, such as psoriasis, atopic dermatitis, seborrheic dermatitis, and vitiligo [3].Therefore, DeadSeaclimate treatment by dermatologists [4]. Thanks to these properties, scientists endeavored to investigate the benefits evaluation.TheresultsshowedthatDSWandrelatedcomplexescanprotectthe skin comprehensively via moisturization, barrier repair, anti-inflammation, and anti-aging. This review is aimed to summarize the reports on the mechanism of action, the skincare propertiesoftheDSWanditsrelatedcomplex, as well as research directions in the future
Table1.MajorelementscompositioninDeadSeawater.
MainElements | Name | DeadSeaWater(mg/L) |
| Na+ | Sodium | 2295 |
| K+ Potassium | 1440 | |
| Ca2+ Calcium | 27,620 | |
| Mg2+ Magnesium | 67,120 | |
| Sr2+ Strontium | 516 | |
| Cl− Chlorine | 2300 | |
| Br− Bromine | 38,000 | |
| SiO2 Silicate | <20 | |
| Li+ Lithium | 30 | |
| Mn2+ Manganese | 6 | |
| Zn2+ Zinc | ≤2 | |
1. Mechanism of Action of DSW
- direct action
Thereisalonghistoryoftryingtoincorporateordirectlyusemineral-rich water for skin care. Massive scientific studies have proved The positive skin conditioning effect asa result of different types, concentrations,andratiosofmineralelements[5]. For example, using hairless mice as experimental subjects, the scientist found that applying magnesium chloridesolutionalonecanaccelerateskinbarrierrestoration[6]. The exocytosis functionof of lipid-containing platelet vesicles within upper epidermal keratinocytes was elevated after using chloride ion carriers [7]. Another experiment found that the application of K+channel blockers inhibited skin recovery, whereas treatment with molecules that open the same channels (or are classified as K+carriers) can accelerate skin recovery by regulating platelet vesicle secretion. This suggested that the alterations of K+channel activity can significantly affect skin barrier homeostasis [8]. Moreover, Ca2+ gradient and signaling are also key for a healthy skin barrier and barrier homeostasis. During aging, as well as in diabetic skin,dysregulatedcalciumsignalingoccursandtheCa2+gradientisflattened[9]. Besides driving keratinocyte differentiation, the Ca2+ gradient also plays a role in cell migration and wound healing [10].In summary,itcanbeassumedthattheabundanceofMg2+,Ca2+, Cl−, and K+in DSW can prominently improve the barrier function of the skin.At the same time,theexperimenthasrevealedthattheapplicationof5%MgCl2 specifically inhibit the TNF-αproduction by epidermal cells, and also the antigen-presenting capacity ofLangerhanscells[11]. Due to the high concentration of Mg2+inDSW, we can conclude that DSW has good anti-inflammation potential.
In addition to different types of elements, the concentrations and ratios of elements can also impose various effects on skin health. For example, Avène Thermal Spring Water, which is low in minerals but rich in bicarbonate and silicates, could serve as a regulator for cell membrane fluidity, antioxidants, and an anti-inflammatory agent [12,13]. Oppositely, the mineral-rich Vichy Thermal Spring Water exhibited more diverse skincare effects, including the increase of stratum corneum peroxidase activity and the promotion of skin homeostasis-related gene expression [14,15]. Thus the unique ionic composition and ratioinDSWmayalsoconferpowerfulpotentialforskincareapplications.
- IndirectAction
In previous research,Ca2+,due to its high inspecting sensitivity,was selected edasthe bio markertorepresenttheiontransportinDSW.No change was observed in the calcium concentration of the culture medium, which suggests that the ions in DSW might not function via transdermal transport [16]. Moderate ionic osmotic stress (MIOS), induced by applying hyper saline material like Dead Sea water and mud,has been proven to have beneficial contributions to skin health. The positive effects include an impact on the modulation of cell-cycle dynamics, which further leads to a stronger epidermal barrier function, skin hydration elevation, and inflammatory response reduction [17].The fundamental principle is that the dissolved mineral salt sact through the induction of the cell osmosis, and participates in osmotic stress mechano-transduction via piezo-electric ion channels [18]. Although these conclusions have been proven by many experiments at the molec-
ular level, the fundamental mechanism remains unknown.To verify the conjecture, Cohen et al. performed experiments on internal ROS-elevated skin cells and organ models. They found that enhanced Nrf2 (nuclear factor erythroid-2- related factor 2) translocation into the nucleus, upregulation of phase-II antioxidant enzymes, and downregulation of NF-κB-related inflammatory proteins(cytokines-1β,IL-8,and caspase-3)are witnessed
afterMIOSexposure.Taken together,MIOS can result in modulating in tracellularROS
generation, which activates the physiological redox homeostasis of the skin and evokes the induction of various biochemical pathways, such as the Nrf2pathway [19].
3. BiologicalFunctionofDSWandRelatedComplexes(Figure1)
- DermatologicalTreatment
Atopic dermatitis, psoriasis, vitiligo, ichthyosis, and granuloma an nulare, are typical chronic skin diseases with a high rate of relapse [20]. The treatment of these diseases often involves the topical use of drugs, such as corticosteroids, antihistamines, immunomodulators, and antibacterial agents, whichcanleadtogreatsideeffectsanddrugdependence[21]. Dead Sea climatotherapy, as an effective, cost-effective, and safer method, has been consolidated in many studies for its therapeutic efficacy [22,23].
In 2000, Elkayam et al. conducted Dead Sea climatotherapy (DSC) in psoriatic arthritis patients [24].Within four weeks, several clinical indicators were measured by dermatologistgists atregularintervalstoevaluatetheefficacy.PASI(PsoriasisAreaandSeverityIndex), patient self-assessment, and the Schober test showed statistically significant improvements after treatment.These variables are valid and reliable in psoriatic arthritis severity definition [25,26]. The thecohortstudyof1718patientswithatopicdermatitisshowedthatover95 % clearance could be achieved in 4 weeks and more after DSC [27]. Also, DSW-containing cream has been demonstrated to improve skin parameters associated with atopic dermatitis in children, particularly in transepidermal water loss (TEWL) and objective severity assessment of atopic dermatitis (OSAAD) values [28]. TEWL measurement is widely used to assess skin barrier function [29] and has a significant correlation with the clinical severity of chronic dermatosis [30]. Plaque psoriasis is the most common form of psoriasis its treatment effect can be partly reflected by the PASI value.Harari et al.foundapositiveeffectonPASIafterDSC, particularly in the early stages of the disease [31]. Previous studies have also revealed increased levels of encephalin, an opioid peptide known to modulate inflammatory responses and keratinocyte proliferation,inpsoriaticskintissues[32]. After four weeks of treatments, the clinical symptoms of patients disappeared and enkephalin levels in keratinocytes decreased by 21% [33]. Meanwhile, the mean SCORAD (atopic dermatitis score) value was found to decrease from 50.5 to 11 after around 30-day DSC treatments [34].The potentialtherapeuticeffectofDSConvitiligowasalsoconfirmedbyanalyzingtheclinical statistical parameters of 436 patients.At the end of treatment, more than 80% of the patients showed improved repigmentation, which was better than the typical narrow-band ultraviolet B treatment [35]. DSC treatment in psoriasis disease can not only bring immediate alleviation but also exert a long-lasting effect [36]. Also, human trials suggested thatthemeanPASIdecreasedfrom31.7to1.42afterthefour-weekDSCtreatmentwithan improvementof95.5%.All patients achieved PASI50,whichwasthoughttobeaclinically meaningful improvement and primary endpoint in psoriasis [37], and the therapeutic effect can last up to 33.6 weeks [38].These results together revealed the good applicate potential of DSW in chronic skin disease treatment.
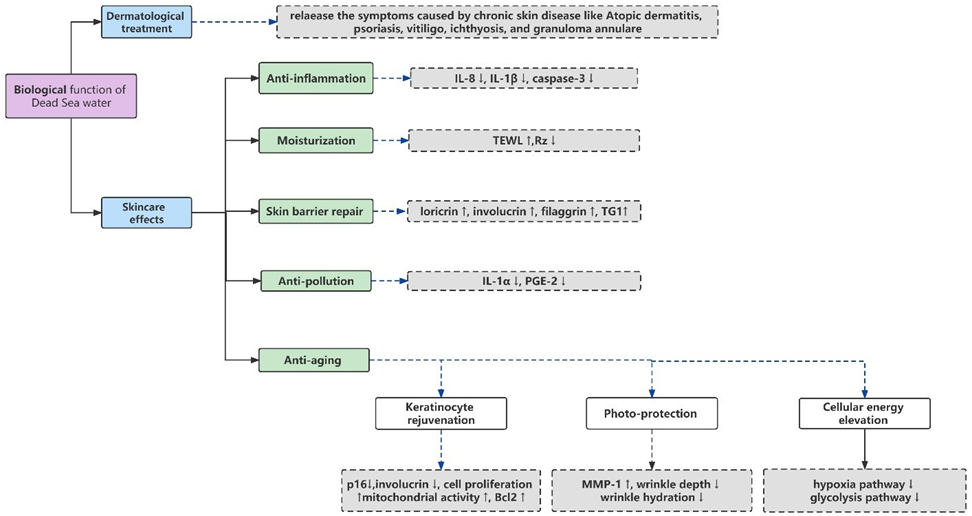
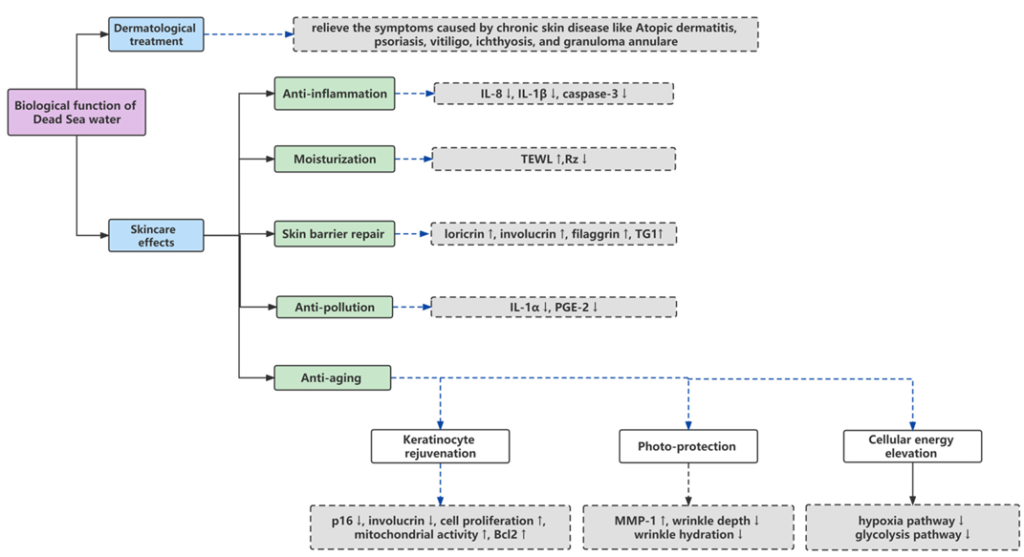
Figure1.OverviewofthebiologicalfunctionofDeadSeawaterindermatologyandskincare.
SkincareEffects
Moisturization
To clarify the effect of bathing in DSW, especially the biophysical characteristics in atopic dry skin, transepidermal water loss (TEWL), skin hydration, skin roughness, and skin redness were measured. Eitan et al.compared the indexes before the study and at weeks 1–6,thentheyobservedtheelevationofbasalTEWLandenhancedskinhydration. Meanwhile, the common skin inflammation markers, such as roughness and redness, were significantly reduced after treatment [39]. By by evaluating skin roughness using computer-aided laser profilometry, Ma’Or et al.investigated the cutaneous smoothing
effects of three different liquid gels,oneofwhichcontainedDeadSeaminerals. After four weeks, the gel containing 1% Dead Sea mineral solution reduced skin roughness by 40.7%, whichboostedabettermoisturizingeffectthantheothertwogels[40].
In addition, the complex esth at combining DSW with other active substances also exhibits good moisturizing properties. Triple D ComplexTM, the mixture of DSW, Dunaliella salina algae extract, and desert plants, was developed and added into a cosmetic cream. Dunaliellasalina,a uni cellular halophilic microalga with ared colony, is the commercialized producer of many compounds within the carotenoid family. Its extract showed good antiglycation, anti-aging, and anti-inflammatory capabilities in the ex vivo human skin explants model [41,42]. After four-week treatments, they found an average reductionoftheskinroughnessparameterRzby43%,which is measured by the silicone impression. Theskinsurfacehydrationstatewasalsoassessedbyacorneometer(askin capacitance-based instrument) and the appearance improvement was also observed after application[43].
Anti-Inflammation
Cohenetal. used UVB irradiation-induced skin explant as the model and co-cultured it with the DSW. A downregulated level of interleukins secretion levels, such as IL-8 and IL-1β, as well as a lower level of caspase-3 proapoptotic enzyme, were observed after DSW treatment [19]. Furthermore, Portugal-Cohen et al. also constructed another inflammation-induced model by lipopolysaccharides (LPS) on Human skin organ culture. They found thattreatmentwithDSWatconcentrationsof0.1%and0.5%cansignificantlyattenuate IL-1βinductionby 46% and 54%, respectively [16].From the in vitro tests, it is obvious that DSW exerts inflammation inhibition ability.
Clinically, the DSW also demonstrated an anti-inflammation effect on chronic inflammatory diseases,such as psoriasisoratopicdermatitis.AsreportedbyProksch[39], magnesium-richDeadSeasaltsolutionwasappliedtopatientswithatopicdryskin.A 15 min treatment in bath solution containing 5% Dead Sea salt for 6 weeks could greatly improve skin hydration, and roughness, and reduce skin redness. It was proposed that the anti-inflammatory property of the DSW might originate from the modulation of interleukins, as well as the antigen-presenting capacity of Langerhans’ cells [44].
Magnesium in DSW might contribute the most to inflammation inhibition. DSW is knownfortheabundanceofmagnesiumions.AssummarizedbyTarnowska[45], magnesium could reduce TNF-αproduction in epidermal cells, thus bringing skin-soothing effects. Additionally, magnesium-rich Dead Sea therapy has been proven to downregulate nuclear factorκB(NFκB)to avoid further inducement of pro-inflammatory factor sand integrin.In
Study [46],a Korean Seaderivativewithsimilarmineralcompositionshowedsimi- lar anti-inflammatory properties, consolidating the hypothesis that magnesium-enriched seawater has a protective effect against skin inflammation from different sources.
SkinBarrierRepair
Skin, as the largest organ in the human body, forms the first barrier against different exogenous stress, performing a barrier function maintenance in skin homeostasis.To clarify the internal mechanism, four barrier function-related proteins: loricrin, involucrin, filaggrin, and transglutaminase 1 (TG1), were measured on skin equivalents after applying DSW at concentrations of 0.8% and 2% [16].The results suggested that the latter three structural-relatedbiomarkerswereupregulatedaftertopicalDSWapplication.Involucrin is thought to be one of the precursors cross-linked during the resistant cornified envelope assembly process, which is partly driven by TG1 [47]. Filaggrinisano an important protein during the final stages of keratinocyte differentiation, which can promote keratin filament aggregation and further form into tight bundles [48]. Further tests on SDS-induced (sodium dodecyl sulfate) human skin irritation organ culture (HSOC) showed that topical DSW application alleviated the reduced epidermal viability and decreased IL-1αand PGE2 levels, which are in line with previous reports of skin response to minerals in animal models.DSW can also attenuateLPS-inducedIL-1βsecretion, which is probably related to its skin barrier- to restoring effect. The complete skin barrier can prevent skin from the harmful stimulation of the external environment and from the release of inflammatory markers, such as cytokine and PGE [49]. Former research has found that osmotic pressure can stimulate TRPV4, an importantsignalmoleculeintheregulationofcalciumgradientandbarrierhomeostasis in the epidermis [50]. Considering the central role of Ca2+in epidermic protein synthesis and its high concentration in DSW, it can be conjectured that DSW might function in two parallel ways, namely the mediation of TRPV4 and the calcium-pump activation by osmotic pressure, thus regulating the expression and activity of the epidermal-related protein.
Anti-Pollution
With the increasing deterioration of the natural environment, the negative skin impact of air pollution attracts the attention of dermatologists [51]. In a recent study, the common pollution models, ozone and a mixture of pollutants (MOP) composed of heavy metal and atmospheric particulate matter [52], were selected to induce the oxidatively-stressed statein3Dskincellculture. Using epidermal viability and inflammatory biomarkers as the indicator of effect, it was found that DSW can inhibit the IL-1αoverproduction following MOP exposure. When mixed with another active ingredient, for example, anionic polysaccharide PolluStop®(bio-saccharide gum-4 or 1,2-hexanediol), the release of IL-1α and PGE-2 induced by ozone exposure can be further reduced [53].
Anti-Aging
Keratinocyte Rejuvenation
Skin aging often leads to increased wrinkles, decreased elasticity, and reduced skin thickness [54]. These phenotypes can not only bring a negative appearance but also deteriorate the confidence. Accordingly, the anti-agingfunctionofDSWwasstudied and verified by a series of experiments. First, the researchers developed the biological model of aged epidermal keratinocytes by characterizing the cellular and molecular properties, including the morphological, fluorometric, and biochemical parameters on both skin cells and organ cultures [55]. Then,thealteredexpressionof16biochemicalmoleculesin both aged cultured cell sand tissues was observed and selected as the aging biomarkers, including caspases-1and3,beta-galactosidases,p16, Ki67,20Sproteasome, and effects of the Fas-dependent apoptotic pathway [56]. After applying DSW to both models, they found that mitochondrial activity and cell proliferation are increased at subtoxic doses. The DSW treatment group also showed significantly reduced p16 and involucrin signals and increased Bcl2 levels, as observed in non-senescent cells.A possible explanation is that DSWcaneliminatepoorlyproliferativeandagedcells, active keratinocyte population.In summary, DSW can stimulate proliferation and mitochondrial activity, decrease the expression of aging biomarkers, and limit apoptotic damage after UVB irradiation. Apart from the use of pure DSW, a combination of DSW and traditional anti-aging actives may present a synergistic effect. Retinol is a commonly-employed ingredient to im- prove age-related skin issues, but its safety profile is controversial.The scientists designed a new complex named“pRetinolTM”(PRE),whichcontainsβ-carotene, niacinamide,the extract of Dunaliellasalina,and DSW.Theformertwocanserveastheprecursorstosynthe-
sizing retinol [57]. The extract of Dunaliella salina alga has been proven to contain multiple anti-antioxidant substances, which include fatty acids and pigments, such as β-carotene and chlorophyll.The biological function of this complex was evaluated and compared with retinol only by using three different models: invitro human dermal fibroblast cell, reconstructed 3D skin equivalent, and ex vivo human skin organ culture. The measurement of hyaluronic acid, TNF-α, and IL-1αexpression levels revealed it is of retinol-like skin activity, yet it led to less skin irritation. The whole-genome microarray was also performed to compare the different expression pathways between PRE complex and retinol treatment. The enrichmentanaly sis showed that PREcanreducemanypathwaysinvolvinginflam-motion,suchasNOD-likereceptorsignaling[58], TNF signaling, nuclear factor-kappa B (NF-κB) signaling [59], and apoptosis.The complex can additionally up-regulate the BASEexcisionrepair-related gene expression. AsanimportantpartoftheoxidativeDNA damage defense mechanism [60], this complex might provide a protective effect on retinol, which has side effects and toxicity [61].
Photo-Protection
Among the multiple exogenous sources that can result in skin photo-damage and aging, exposure is the most important and well-known factor[62]. AmixtureofDSW and three plants (Tibetangojiberry, Himalayanraspberryrootextract, and Iceland moss (lichen)), namely Extreme ComplexTM, was developed in the previous report [63].The UVB- induced ex vivo human skin model was reconstructed and used to assess its protection function against light radiation. A reduced caspase-3activity and pro-inflammatory cytokine TNF-αsecretionwere shown, which suggested its anti-apoptotic and anti-inflammatory functions [64]. Additionally, the complex can also decrease the activity of collagen balance- related biomarkers, degrading enzymes, and collagen maturation by-products. Within the matrix metalloproteinase (MMP) family, MMP-1 is the most injured enzyme in collagen damagewhenfacingphotoaging[65]. MMP-1activationcanfurtherinducetheincreased expressionlevelofMMP-3andMMP-9,acceleratingcollagendegradation[66]. In clinical tests on human subjects,skin wrinkle depth and hydration we recommend hat can reflect the degree of skin photoaging, which can be measured using the PRIMOS optical 3D measuring device and chronometer, respectively [67].A significant improvement in skin moisture and wrinkle depth is observed after application.Altogether, the antioxidant, anti-apoptotic, and anti-inflammatory characteristics of this complex might bring comprehensive alleviation effects of skin photo-damage and appearance improvement.
Under certain conditions, adding metal ions into the fermentation medium has been proven to improve the structure and function of products [68]. Since Dead Sea water is rich in minerals and trace elements, scientists developed a fermentation supplement by mixing the water and mud of the Dead Sea, which acts as a positive stress supplement during yeastPichiapastoris(akaKomagataellaphaffii)fermentation. This kind of methylotrophic microorganism is one of the most commonly used cell factories for heterologous protein production and has been used in many industries [69].To evaluate the biological functions of this complex, Portugal-Cohenetal.first measured a series of skin elasticity biomarkers on3Dhumanskinequivalentsandperformedthewhole-genomeDNAmicroarraytestat the same time,to investigate its effect on both gene and protein levels, respectively[70]. After treatment, the experimental group had significant alleviation of abnormal UVB-induced alterations; for example, both elastin and fibulin are the main components of the extracellular matrix, which is the damage target of UV solar [71]. Tight junction protein (TJ) can connect multiple parallel intramembranestrandsandneigh boring cells into are gulatory and structural network [72], which can contribute to the skin’s UV resistance capacity [73]. The elevation of these proteins indicates the skincare potential of this complex. The UV-protection effect of DSW is also consolidated in DSW-containing cosmetic creams[74].AcreamcomposedofDSW, zinc oxide, aloe vera extract, pro-vitamin B5, and vitaminE, was formulated and topically applied to human skin organ cultures exposed to UVB.AseveremitochondrialactivitylosswasobservedafterUVradiation, as activated by caspase-3 and cytokine secretion, which was well accorded with previous studies [75]. On the contrary, the DSW-incorporated formulation significantly promoted the anti-oxidative capacity and reduced cell damage and apoptosis.It was inferred that the DSW could minimize oxidative stress and inflammatory signs after UV exposure.
CellularEnergyElevation
Organisms that live in extreme environments usually evolve many special mechanisms for survival [76]. Calotropis procera, the traditional American medicinal plant, naturally grows in the flora of the Dead Sea region [77]. The previous study has found many pharmacological actions of its extracts, such as anti-inflammatory, antibacterial, and antioxidant [78]. After mixing with DSW, various biological activities are further amplified and enhanced, which was verified by the RNA microarray experiment on the reconstructed full-thickness skin tissues. The GSEA analysis results showed that the biological processes of hypoxia, glycolysis, and epithelial-mesenchymal transition pathway were significantly down-regulated after treatment. These results indicated that this complex had an unexpected biological potential for energy production, resistance hypoxia, and ECM balance [79].
4.CurrentChallengesfortheUseofDSW
Though DSW shows a unique biological role in maintaining skin health, there are still tremendous issues to be addressed.
ThecoreproblemishowtostrikethebalancebetweenDeadSearesourcesexploitation andeco-protection.Environmental factors, such as climate alteration, ongoing sinkholes, and geochemistry variation, could lead to the scarcity of water resources [80].In addition to natural influences,humaninterventionalsothreatensthepreservationofDeadSea resources. Water pollution, exhaustive exploitation, and changes in biodiversity could all aggravatetheexhaustionofDeadSearesources. As reported,theDeadSea’ssealevelhas droppedataspeedof1m/year in the last 5 decades, bringing the challenge of sustainable supply of DSW.
TheexteriorvariationoftheDeadSeaalsocontributestoanotherissue: quality control. Apart from seasonal and locational differentiation, the ion concentration, the ratio between different minerals, and the metabolites from microbial could be affected by environmental changes, which might eventually lead to unpredictable biological effects.
The application of DSW in cosmetic products also evokes great challenges, not only because a higher concentration of DSW in products might cause potential skin irritation and discomfort, but also due to its relatively high ion strength. This would hamper the stability of the cosmetic formula by sabotaging emulsion thermo-dynamic homogeneity and altering the rheological properties of the whole system. Additionally, DSW might also impose an antagonistic effect with other actives in the formula, resulting in unwanted precipitation or efficacy invalidation. However, there is a cosmetic brand that has produced a series of related products with broad effects and diverse forms, like hydrating sprays, lotions, and creams. The DSW here functions more as the actives instead of the main ingredient.
Considering this, many efforts have been made to improve their utilization in formulations.For example, scientists dispersed the nanosized Dead Sea mineral in mixed oil (Crystal Osmoter™) and achieved six times higher concentrations of DSW on the skin, for which the clinical tests showed a better performance on wrinkle reduction, firming, and radiance than normal DSW with no irritation.At the same time, new delivery systems were also developed, like liposomes (LipOsmoter™) or strontium hexaferrite (SrFe12O19) nanomagnets, which enhance the safety of DSW and provide a longer-term skincare benefit. AlthoughtherearesomeinventionsabouttheDSWapplicationincosmeticsproducts,
still many issues need to be explored,likehowtocomplementthesynergyofDSWand how to stabilize the system even if added in a high DSW concentration.
Since the DSW is a complex mixture of different minerals and other trace elements, itisofgreaturgencytodeterminetheexactcompositionofDSW.Especiallyforthetrace elements, more advanced analytical techniques are needed to quantitatively evaluate their contents. Facing resource shortages and environmental pollution, the artificial DSW (ADSW) consisting of the main ion compositions are formulated in labs. Unfortunately, it has shown weaker effects on both cell models and human skin organ cultures than natural DSW (unpublished data). Itisprobablethatthetraceelementandmicrobe-secreted bioactive substancescanmakeuppartoftheskincareeffectsofnaturalDSW.Thus a more delicate choice of DSW content should be studied first.
In addition, the interaction between different ions and the dose-function relation of DSW should be further investigated. Each type of ion might play complex roles in modulating biological processes and molecular functions on the skin, thus DSW might bring even more complicated cellular interplays. To sum up, the current challenges for the use of DSW lie in the short supply, unpredictable quality variation, compatibility concerns in the formulas, as well as lack of understanding of DSW components and interrelated mechanisms.
5. FutureDirection
The skincare benefits of Dead Sea water and its related complex have been demonstrated in many studies and cover a wide range of functions, including moisturization, barrier repair,anti-inflammation,anti-aging,photo-protection, cellular energy elevation, and relief of skin disease symptoms. However, several issues are still worthy of further investigation, which are listed below.
Firstly, although the extreme hypersaline environmental conditions of Dead Sea water hamper the growth of higher plants and animals, microorganisms like fungi and bacteria can still survive [81–83].Extremophilesareoftenthoughttobeahugereservoirofactive substances [84]. For example, an extremely salt-tolerant Bacillus strain isolated from the Dead Sea had significant antibacterial and fungal activity in its aqueous extract. One of them can evenresistallthetestedbacteriastrains[85,86].Haloarculavallismortisisakindofhalophilic archaeon with reddish colonies [87]. Its extract has a significant anti-inflammatory activity that can resist the DNA damage induced by UV exposure,whichsuggestsitspotentialfor use as a biological or natural sunscreen[88]. Although some Dead Sea source trains have been isolated and purified, studies for their bioactive product utilization are still scarce and need to be further investigated experimentally.
Secondly, the number of skin microbiome-related studies is increased rapidly in recent years and the relationship between the skin microbiome and skin health is gradually being revealed [89–91]. The effects of DSW and related complexes on skin microbiomes are less evaluated, and most of them are involved in skin diseases. Based on a healthy population, a previous experiment compared the change in the skin microbiome after DSW application[83]. They found that bacterial community diversity is almost constant, whilefungaldiversitywassignificantlylowerthanbefore,the variation of which is driven primarily by Malassezia spp.It’s known that the abnormal abundance of Malassezia can leadtofreeradicalsreleaseandinflammatoryskinissues[92].The variation pattern of AD (Atopic Dermatitis) patients has also been explored after using DSW. The results show that the unbalance of skin microbial ecology, which occurs at both lesion and non-lesion sites, was significantly attenuated after DSW application [93]. The most significant changes were seen in severe,mainlyreflectedintherelativeabundanceofStaphylococcus
epidermidis, Streptococcus mitis,andMicrococcusluteus.Current studies usually used the high- the high-throughput 16S rRNA or ITS (internally transcribed spacer) amplicon sequencing method, which is of low resolution and limited information availability.A more comprehensive metagenome approach should be established subsequently to obtain more accurate and profound findings [94].
Thirdly,incontrasttotheextensiveexplorationofDSW, the research involved in the skincare effect of Dead Sea mud is rare [2,95–98]. Previous experimental results have proventhatbothshort-termandlong-termapplicationofDeadSea-derivedmudhasa high safety profile. It causes no damage to skin barrier integrity but has a firming effect instead [99,100]. Furthermore, Dead Sea black mud-derived masks have been shown to accelerate the wound healing process in mice skin by promoting granulation, angiogenesis, and collagen deposition [101].The mud is also found to inactivate common microorganisms and produce an obvious growth inhibition area, suggesting its significant antibacterial effect [102]. As the seed of Dead Sea minerals, the studies of Dead Sea mud should not be limited to the basic skincare functions, but also more toits applications in cosmetics.
6. Conclusions
Dead Sea water has a unique ion composition and its benefits on skin health have been well-known since ancient times. From previous research, we summarize two potential action modes of DSW. The first one is the direct penetration of mineral ions, and the second one is the moderate ionic osmotic stress mechanism, which can activate the cellular osmoticstress-relatedpathwayviaionchannels.Thechronicskindiseaseimprovements andcomprehensiveskincareefficacyofDSWanditsrelatedcomplexarealsoillustrated. Specifically, they can resist skin senescence from three different perspectives (keratinocyte rejuvenation promotion, photo-protection, and cellar energy elevation), which indicates their strong application potential in anti-aging cosmetics product development.However, many other aspects of the Dead Sea resource are still unknown and need to be studied, suchastheDeadSeamud,secondarymetabolitesofDeadSeabacteriaandfungi,andalso their effects on the skin microbiome.
AuthorContributions:Conceptualization,D.D.andX.M.;writing—originaldraftpreparation,D.D; writing—review and editing, D.D., X.M., X.Y. and X.B. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no externalfunding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
DataAvailabilityStatement:Notapplicable.
ConflictsofInterest:Theauthorsdeclarenoconflictofinterest
References
1. Even-Paz, Z.; Shani, J. The Dead Sea and psoriasis: Historical and geographic background. Int. J. Dermatol. 1989, 28, 1–9.[CrossRef] [PubMed]
2. Buskila, D.; Abu-Shakra, M.; Neumann, L.; Odes, L.; Shneider, E.; Flusser, D.; Sukenik, S. Balneotherapy for fibromyalgia at the Dead Sea. Rheumatol. Int. 2001, 20, 105–108. [CrossRef] [PubMed]
3. Yao, Y.; Ravn Jørgensen, A.-H.; Thomsen, S.F. Biologics for chronic inflammatory skin diseases: An update for the clinician. J. Dermatol. Treat. 2020, 31, 108–130. [CrossRef] [PubMed]
4.Huang, A.; Seité, S.; Adar, T. The use of balneotherapy in dermatology. Clin. Dermatol. 2018, 36, 363–368. [CrossRef]
5. Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P. The role of thermal water in chronic skin diseases management: A review of the literature. J. Clin. Med. 2020, 9, 3047. [CrossRef]
6. Maarouf, M.; Hendricks, A.J.; Shi, V.Y. Bathing additives for atopic dermatitis—A systematic review. Dermatitis 2019, 30, 191–197. [CrossRef]
7. Denda, M.; Fuziwara, S.; Inoue, K. Influx of calcium and chloride ions into epidermal keratinocytes regulates exocytosis of epidermal lamellar bodies and skin permeability barrier homeostasis. J. Investig. Dermatol. 2003, 121, 362–367. [CrossRef]
8. Bäsler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 3–14. [CrossRef]
9. Hudson, L.E.M. Integration of Wound-Induced Calcium Signals to Transcriptional Activation and Regulation of Cutaneous Wound Healing Responses; Newcastle University: Tyne, UK, 2015.
- Lee, S.E.; Lee, S.H. Skin barrier and calcium. Ann. Dermatol. 2018, 30, 265–275. [CrossRef]
- Schempp, C.M.; Dittmar, H.C.; Hummler, D.; Simon-Haarhaus, B.; Schöpf, E.; Simon, J.C.; Schulte-Mönting, J. Magnesium
ions inhibit the antigen-presenting function of human epidermal Langerhans cells in vivo and in vitro. Involvement of ATPase,
HLA-DR, B7 molecules, and cytokines. J. Investig. Dermatol. 2000, 115, 680–686. [CrossRef] - Eliasse, Y.; Redoules, D.; Espinosa, E. Impact of Avène Thermal Spring Water on immune cells. J. Eur. Acad. Dermatol. Venereol.
2020, 34, 21–26. [CrossRef] [PubMed] - Nocera, T.; Jean-Decoster, C.; Georgescu, V.; Guerrero, D. Benefits of Avène thermal hydrotherapy in chronic skin diseases and
dermatological conditions: An overview. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 49–52. [CrossRef] [PubMed] - Tacheau, C.; Weisgerber, F.; Fagot, D.; Bastien, P.; Verdier, M.; Liboutet, M.; Sore, G.; Bernard, B. Vichy Thermal Spring Water
(VTSW), a cosmetic ingredient of potential interest in the frame of skin ageing exposome: An in vitro study. Int. J. Cosmet. Sci.
2018, 40, 377–387. [CrossRef] [PubMed] - Rasmont, V.; Valois, A.; Gueniche, A.; Sore, G.; Kerob, D.; Nielsen, M.; Berardesca, E. Vichy volcanic mineralizing water has
unique properties to strengthen the skin barrier and skin defenses against exposome aggressions. J. Eur. Acad. Dermatol. Venereol.
2022, 36, 5–15. [CrossRef] - Portugal-Cohen, M.; Cohen, D.; Ish-Shalom, E.; Laor-Costa, Y.; Ma’or, Z.e. Dead Sea minerals: New findings on skin and the
- biology beyond. Exp. Dermatol. 2019, 28, 585–592. [CrossRef]
- Levi-Schaffer, F.; Shani, J.; Politi, Y.; Rubinchik, E.; Brenner, S. Inhibition of proliferation of psoriatic and healthy fibroblasts in cell
culture by selected Dead-sea salts. Pharmacology 1996, 52, 321–328. [CrossRef] - Carbajo, J.M.; Maraver, F. Salt water and skin interactions: New lines of evidence. Int. J. Biometeorol. 2018, 62, 1345–1360.
[CrossRef] - Cohen, D.; Ma’or, Z.e.; Cohen, M.P.; Oron, M.; Kohen, R. Nrf2 Pathway Involvement in the Beneficial Skin Effects of Moderate
Ionic Osmotic Stress–the Case of the Dead Sea Water. J. Cosmet. Dermatol. Sci. Appl. 2022, 12, 109–130. - Sawada, Y.; Saito-Sasaki, N.; Mashima, E.; Nakamura, M. Daily Lifestyle and Inflammatory Skin Diseases. Int. J. Mol. Sci. 2021,
22, 5204. [CrossRef] - Duong,T.A.; Valeyrie-Allanore, L.; Wolkenstein, P.; Chosidow, O. Severe cutaneous adverse reactions to drugs. Lancet 2017, 390,
1996–2011. [CrossRef] - Marsakova, A.; Kudish, A.; Gkalpakiotis, S.; Jahn, I.; Arenberger, P.; Harari, M. Dead Sea climatotherapy versus topical steroid
treatment for atopic dermatitis children: Long-term follow-up study. J. Dermatol. Treat. 2019, 31, 711–715. [CrossRef] [PubMed] - Emmanuel, T.; Petersen, A.; Houborg, H.I.; Rønsholdt, A.B.; Lybæk, D.; Steiniche, T.; Bregnhøj, A.; Iversen, L.; Johansen, C.
Climatotherapy at the Dead Sea for psoriasis is a highly effective anti-inflammatory treatment in the short term: An immunohis
tochemical study. Exp. Dermatol. 2022, 31, 1136–1144. [CrossRef] [PubMed] - Elkayam, O.; Ophir, J.; Brener, S.; Paran, D.; Wigler, I.; Efron, D.; Even-Paz, Z.; Politi, Y.; Yaron, M. Immediate and delayed effects
of treatment at the Dead Sea in patients with psoriatic arthritis. Rheumatol. Int. 2000, 19, 77–82. [CrossRef] - Bo˙ zek, A.; Reich, A. The reliability of three psoriasis assessment tools: Psoriasis area and severity index, body surface area and
physician global assessment. Adv. Clin. Exp. Med. 2017, 26, 851–856. [CrossRef] - Elewski, B.E.; Puig, L.; Mordin, M.; Gilloteau, I.; Sherif, B.; Fox, T.; Gnanasakthy, A.; Papavassilis, C.; Strober, B.E. Psoriasis
patients with psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements
than those with PASI 75–89 response: Results from two phase 3 studies of secukinumab. J. Dermatol. Treat. 2017, 28, 492–499.
[CrossRef] - Harari, M.; Shani, J.; Seidl, V.; Hristakieva, E. Climatotherapy of atopic dermatitis at the Dead Sea: Demographic evaluation and
cost-effectiveness. Int. J. Dermatol. 2000, 39, 59–69. [CrossRef] [PubMed] - Portugal-Cohen, M.; Oron, M.; Merrik, E.; Ben-Amitai, D.; Yogev, H.; Zvulunov, A. A dead sea water-enriched body cream
improves skin severity scores in children with atopic dermatitis. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 71. [CrossRef] - Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research techniques made simple: Transepidermal water loss measurement as a
research tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [CrossRef] - Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández
González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water
loss and temperature as useful tools to assess disease severity. J. Clin. Med. 2021, 10, 359. [CrossRef] - Harari, M.; Czarnowicki, T.; Fluss, R.; Ruzicka, T.; Ingber, A. Patients with early-onset psoriasis achieve better results following
Dead Sea climatotherapy. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 554–559. [CrossRef] - Bigliardi, P.L.; Bigliardi-Qi, M.; Buechner, S.; Rufli, T. Expression of-opiate receptor in human epidermis and keratinocytes. J.
Investig. Dermatol. 1998, 111, 297–301. [CrossRef] [PubMed] - Nissen, J.; Avrach, W.; Hansen, E.; Stengaard-Pedersen, K.; Kragballe, K. Increased levels of enkephalin following natural sunlight
(combined with salt water bathing at the Dead Sea) and ultraviolet A irradiation. Br. J. Dermatol. 1998, 139, 1012–1019. [CrossRef]
[PubMed] - Harari, M.; Dreiher, J.; Czarnowicki, T.; Ruzicka, T.; Ingber, A. SCORAD 75: A new metric for assessing treatment outcomes in
atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1510–1515. [CrossRef] [PubMed] - Czarnowicki, T.; Harari, M.; Ruzicka, T.; Ingber, A. Dead Sea climatotherapy for vitiligo: A retrospective study of 436 patients. J.
Eur. Acad. Dermatol. Venereol. 2011, 25, 959–963. [CrossRef] [PubMed] - Emmanuel,T.; Lybæk, D.; Johansen, C.; Iversen, L. Effect of Dead Sea climatotherapy on psoriasis; a prospective cohort study.
Front. Med. 2020, 7, 83. [CrossRef] [PubMed] - Carlin, C.S.; Feldman, S.R.; Krueger, J.G.; Menter, A.; Krueger, G.G. A 50% reduction in the Psoriasis Area and Severity Index
(PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J. Am. Acad. Dermatol. 2004, 50, 859–866. [CrossRef]
[PubMed] - Harari, M.; Novack, L.; Barth, J.; David, M.; Friger, M.; Moses, S.W. The percentage of patients achieving PASI 75 after 1 month
and remission time after climatotherapy at the Dead Sea. Int. J. Dermatol. 2007, 46, 1087–1091. [CrossRef] - Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier
function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157. [CrossRef] - Ma’Or, Z.; Yehuda, S.; Voss, W. Skin smoothing effects of Dead Sea minerals: Comparative profilometric evaluation of skin
surface. Int. J. Cosmet. Sci. 1997, 19, 105–110. [CrossRef] - Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella salina Extract Counteracts Skin
Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104.
[CrossRef] - Xu,Y.; Harvey, P.J. Carotenoid production by Dunaliella salina under red light. Antioxidants 2019, 8, 123. [CrossRef] [PubMed]
- Ma’Or, Z.; Meshulam-Simon, G.; Yehuda, S.; Gavrieli, J.; Sea, D. Antiwrinkle and skin-moisturizing effects of a mineral-algal
botanical complex. J. Cosmet. Sci. 2000, 51, 27–36. - Wang, B.; Amerio, P.; Sauder, D.N. Role of cytokines in epidermal Langerhans cell migration. J. Leukoc. Biol. 1999, 66, 33–39.
[CrossRef] [PubMed] - Tarnowska, M.; Briançon, S.; Resende de Azevedo, J.; Chevalier, Y.; Bolzinger, M.A. Inorganic ions in the skin: Allies or enemies?
Int. J. Pharm. 2020, 591, 119991. [CrossRef] [PubMed] - Kim, J.H.; Lee, J.; Lee, H.B.; Shin, J.H.; Kim, E.K. Water-retentive and anti-inflammatory properties of organic and inorganic
substances from Korean sea mud. Nat. Prod. Commun. 2010, 5, 395–398. [CrossRef] [PubMed] - Sevilla, L.M.; Nachat, R.; Groot, K.R.; Klement, J.F.; Uitto, J.; Djian, P.; Määttä, A.; Watt, F.M. Mice deficient in involucrin,
envoplakin, and periplakin have a defective epidermal barrier. J. Cell Biol. 2007, 179, 1599–1612. [CrossRef] - Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [CrossRef]
- Song,C.; Liu, L.; Chen, J.; Hu, Y.; Li, J.; Wang, B.; Bellusci, S.; Chen, C.; Dong, N. Evidence for the critical role of the PI3K signaling
pathway in particulate matter-induced dysregulation of the inflammatory mediators COX-2/PGE2 and the associated epithelial
barrier protein Filaggrin in the bronchial epithelium. Cell Biol. Toxicol. 2020, 36, 301–313. [CrossRef] - Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.;
Torres-Quiroz, F. TRPV4: A physio and pathophysiologically significant ion channel. Int. J. Mol. Sci. 2020, 21, 3837. [CrossRef] - Richard, F.; Creusot, T.; Catoire, S.; Egles, C.; Ficheux, H. Mechanisms of pollutant-induced toxicity in skin and detoxification:
Anti-pollution strategies and perspectives for cosmetic products. In Annales Pharmaceutiques Françaises; Elsevier: Amsterdam,
The Netherlands, 2019. - McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging—Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137.
[CrossRef] - Portugal-Cohen, M.; Oron, M.; Cohen, D.; Ma’or, Z. Antipollution skin protection–a new paradigm and its demonstration on two
active compounds. Clin. Cosmet. Investig. Dermatol. 2017, 10, 185. [CrossRef] [PubMed] - Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. PharmaTutor 2019, 7, 22–70.
- Soroka, Y.; Ma’or, Z.; Leshem, Y.; Verochovsky, L.; Neuman, R.; Brégégère, F.M.; Milner, Y. Aged keratinocyte phenotyping:
Morphology, biochemical markers and effects of Dead Sea minerals. Exp. Gerontol. 2008, 43, 947–957. [CrossRef] [PubMed] - Chervonsky, A.V. Apoptotic and effector pathways in autoimmunity. Curr. Opin. Immunol. 1999, 11, 684–688. [CrossRef]
- Kara´zniewicz-Łada, M.; Główka, A. A review of chromatographic methods for the determination of water-and fat-soluble
vitamins in biological fluids. J. Sep. Sci. 2016, 39, 132–148. [CrossRef] - Danis, J.; Mellett, M. Nod-like receptors in host defence and disease at the epidermal barrier. Int. J. Mol. Sci. 2021, 22, 4677.
[CrossRef] - Wang,Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-B signaling in skin aging. Mech. Ageing Dev. 2019, 184,
- [CrossRef]
- Lee, T.-H.; Kang, T.-H. DNA oxidation and excision repair pathways. Int. J. Mol. Sci. 2019, 20, 6092. [CrossRef]
- Cohen, D.; Portugal-Cohen, M. Safe Retinol-Like Skin Biological Effect by a New Complex, Enriched with Retinol Precursors. J.
Cosmet. Dermatol. Sci. Appl. 2020, 10, 59. - Wang,M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven
Photoaging. Oxidative Med. Cell. Longev. 2019, 2019, 8135985. [CrossRef] - Wineman, E.; Portugal-Cohen, M.; Soroka, Y.; Cohen, D.; Schlippe, G.; Voss, W.; Brenner, S.; Milner, Y.; Hai, N.; Ma’or, Z.
Photo-damage protective effect of two facial products, containing a unique complex of Dead Sea minerals and Himalayan actives.
J. Cosmet. Dermatol. 2012, 11, 183–192. [CrossRef] [PubMed] - Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular mechanisms of UV-induced apoptosis and its effects on skin
residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [CrossRef] [PubMed] - Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective
on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [CrossRef] [PubMed] - Gutop, E.; Diatlova, A.; Linkova, N.; Orlova, O.; Trofimova, S.; Khavinson, V. Aging of skin fibroblasts: Genetic and epigenetic
factors. Adv. Gerontol. Uspekhi Gerontol. 2019, 32, 908–914. - Cook, M.K.; Kaszycki, M.A.; Richardson, I.; Taylor, S.L.; Feldman, S.R. Comparison of two devices for facial skin analysis. J.
Cosmet. Dermatol. 2022, 21, 7001–7006. [CrossRef] - Overy, D.; Correa, H.; Roullier, C.; Chi, W.-C.; Pang, K.-L.; Rateb, M.; Ebel, R.; Shang, Z.; Capon, R.; Bills, G. Does osmotic stress
affect natural product expression in fungi? Mar. Drugs 2017, 15, 254. [CrossRef] - Juturu, V.; Wu, J.C. Heterologous protein expression in Pichia pastoris: Latest research progress and applications. ChemBioChem
2018, 19, 7–21. [CrossRef] - Portugal-Cohen, M.; Dominguez, M.F.; Oron, M.; Holtz, R. Dead Sea minerals-induced positive stress as an innovative resource
for skincare actives. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 22. [CrossRef] - Eckersley, A.; Ozols, M.; O’Connor, C.; Bell, M.; Sherratt, M.J. Predicting and characterising protein damage in the extracellular
matrix. J. Photochem. Photobiol. 2021, 7, 100055. [CrossRef] - Heinemann, U.; Schuetz, A. Structural features of tight-junction proteins. Int. J. Mol. Sci. 2019, 20, 6020. [CrossRef]
- Seo, S.H.; Kim, S.-E.; Lee, S.E. ER stress induced by ER calcium depletion and UVB irradiation regulates tight junction barrier
- integrity in human keratinocytes. J. Dermatol. Sci. 2020, 98, 41–49. [CrossRef] [PubMed]
- Portugal-Cohen, M.; Soroka, Y.; Ma’or, Z.; Oron, M.; Zioni, T.; Brégégère, F.M.; Neuman, R.; Kohen, R.; Milner, Y. Protective effects
of a cream containing Dead Sea minerals against UVB-induced stress in human skin. Exp. Dermatol. 2009, 18, 781–788. [CrossRef]
[PubMed] - Birch-Machin, M.A.; Russell, E.V.; Latimer, J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure
and oxidative stress. Br. J. Dermatol. 2013, 169, 9–14. [CrossRef] - Singh, P.; Jain, K.; Desai, C.; Tiwari, O.; Madamwar, D. Microbial community dynamics of extremophiles/extreme environment.
In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–332. - Yaniv, Z.; Koltai, H. Calotropis procera, Apple of Sodom: Ethnobotanical review and medicinal activities. Isr. J. Plant Sci. 2018,
65, 55–61. [CrossRef] - Imosemi, I.O. Evaluation of the toxicity, medicinal use and pharmacological actions of Calotropis procera. Ejpmr 2016, 3, 28–36.
- Portugal-Cohen, M.; Ish-Shalom, E.; Mallon, R.; Corral, P.; Michoux, F. Apple of Sodom (Calatropis procera) callus extract, a novel
skincare active and its biological activity in skin models when combined with Dead Sea water. J. Cosmet. Dermatol. Sci. Appl. 2018,
8, 73–91. [CrossRef] - Kottmeier, C.; Agnon, A.; Al-Halbouni, D.; Alpert, P.; Corsmeier, U.; Dahm, T.; Eshel, A.; Geyer, S.; Haas, M.; Holohan, E.;
et al. New perspectives on interdisciplinary earth science at the Dead Sea: The DESERVE project. Sci. Total Environ. 2016, 544,
1045–1058. [CrossRef] [PubMed] - Anton, B.P.; DasSarma, P.; Martinez, F.L.; DasSarma, S.L.; Al Madadha, M.; Roberts, R.J.; DasSarma, S. Genome Sequence of
Salarchaeum sp. strain JOR-1, an extremely halophilic archaeon from the Dead Sea. Microbiol. Resour. Announc. 2020, 9, e01505-19.
[CrossRef] [PubMed] - Yin,W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423.
[CrossRef] - Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15,
285–296. [CrossRef] - Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. A perspective on biotechnological applications of thermophilic microalgae
and cyanobacteria. Bioresour. Technol. 2019, 278, 424–434. [CrossRef] [PubMed] - Obeidat, M. Isolation and characterization of extremely halotolerant Bacillus species from Dead Sea black mud and determination
of their antimicrobial and hydrolytic activities. Afr. J. Microbiol. Res. 2017, 11, 1303–1314. - Al-Karablieh, N. Antimicrobial Activity of Bacillus Persicus 24-DSM Isolated from Dead Sea Mud. Open Microbiol. J. 2017, 11,
372–383. [CrossRef] - Oren, A.; Ginzburg, M.; Ginzburg, B.; Hochstein, L.; Volcani, B. Haloarcula marismortui (Volcani) sp. nov., nom. rev., an extremely
halophilic bacterium from the Dead Sea. Int. J. Syst. Evol. Microbiol. 1990, 40, 209–210. [CrossRef] [PubMed] - Kim,J.H.; Shin, J.Y.; Hwang, S.J.; Kim, Y.S.; Kim, Y.M.; Gil, S.Y.; Jin, M.H.; Lee, S.H. Effect of Halophilic bacterium, Haloarcula
vallismortis, extract on UV-induced skin change. J. Soc. Cosmet. Sci. Korea 2015, 41, 341–350. - Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and its Interplay with the Environment. Am. J. Clin. Dermatol.
2020, 21, 4–11. [CrossRef] - Dimitriu, P.A.; Iker, B.; Malik, K.; Leung, H.; Mohn, W.; Hillebrand, G.G. New insights into the intrinsic and extrinsic factors that
shape the human skin microbiome. MBio 2019, 10, e00839-19. [CrossRef] - Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A.
The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming.
World Allergy Organ. J. 2017, 10, 29. [CrossRef] - Dawson,T.L., Jr. Malassezia: The forbidden kingdom opens. Cell Host Microbe 2019, 25, 345–347. [CrossRef]
- Brandwein, M.; Fuks, G.; Israel, A.; Sabbah, F.; Hodak, E.; Szitenberg, A.; Harari, M.; Steinberg, D.; Bentwich, Z.; Shental, N. Skin
microbiome compositional changes in atopic dermatitis accompany Dead Sea climatotherapy. Photochem. Photobiol. 2019, 95,
1446–1453. [CrossRef] - Chng, K.R.; Tay, A.S.L.; Li, C.; Ng, A.H.Q.; Wang, J.; Suri, B.K.; Matta, S.A.; McGovern, N.; Janela, B.; Wong, X.F.C.C. Whole
metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016, 1, 1–10.
[CrossRef] [PubMed] - Sukenik, S.; Giryes, H.; Halevy, S.; Neumann, L.; Flusser, D.; Buskila, D. Treatment of psoriatic arthritis at the Dead Sea. J.
Rheumatol. 1994, 21, 1305–1309. [PubMed] - Chadzopulu, A.; Adraniotis, J.; Theodosopoulou, E. The therapeutic effects of mud. Prog. Health Sci. 2011, 1, 132–136.
- Sukenik, S.; Buskila, D.; Neumann, L.; Kleiner-Baumgarten, A. Mud pack therapy in rheumatoid arthritis. Clin. Rheumatol. 1992,
11, 243–247. [CrossRef] [PubMed] - Codish, S.; Abu-Shakra, M.; Flusser, D.; Friger, M.; Sukenik, S. Mud compress therapy for the hands of patients with rheumatoid
arthritis. Rheumatol. Int. 2005, 25, 49–54. [CrossRef] - Hamed,S.; Almalty, A.-M. Skin Tolerance of Three Types of Dead Sea Mud on Healthy Skin: A Short-Term Study. J. Cosmet. Sci.
2018, 69, 269–278 - Hamed, S.; Almalty, A.M.; Alkhatib, H.S. The cutaneous effects of long-term use of Dead Sea mud on healthy skin: A 4-week
- study. Int. J. Dermatol. 2021, 60, 332–339. [CrossRef]
- Abu-Al-Basal, M.A. Histological evaluation of the healing properties of Dead Sea black mud on full-thickness excision cutaneous
wounds in BALB/c mice. Pak. J. Biol. Sci. PJBS 2012, 15, 306–315. [CrossRef] - Ma’or, Z.; Henis, Y.; Alon, Y.; Orlov, E.; Sørensen, K.B.; Oren, A. Antimicrobial properties of Dead Sea black mineral mud. Int. J.
Dermatol. 2006, 45, 504–511. [CrossRef]







