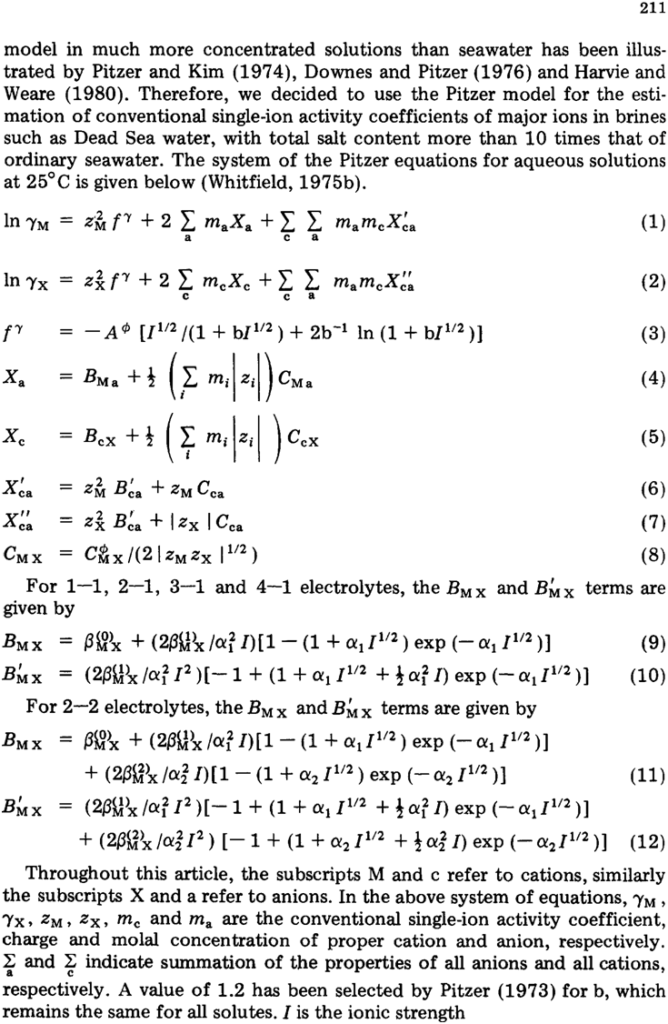ABSTRACT
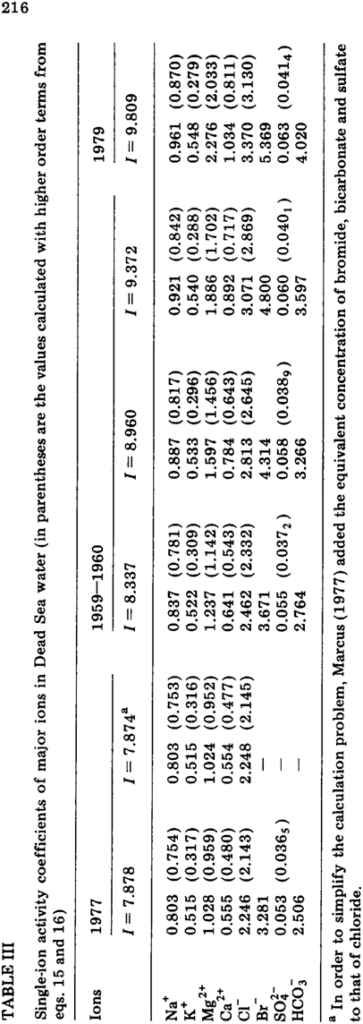
Krumgalz, B.S. and Millero, F.J., 1982. Physico-chemical study of the Dead Sea waters. I. Activity coefficients of major ions in Dead Sea water. Mar. Chem., 11 : 209–222. Conventional single-ion activity coefficients of major ions in Dead Sea brines, and their osmotic coefficients, have been estimated using Pitzer’s thermodynamic treatment of mixed electrolyte solutions. For these calculations, higher-order terms have been taken into consideration. It has been shown that Pitzer’s equations are applicable to such natural concentrated brines. The problem of gypsum solubility in the Dead Sea was examined. Dead Sea brines were shown to be oversaturated with respect to gypsum, and the degree of oversaturation increased very significantly during the last 20 years.
INTRODUCTION
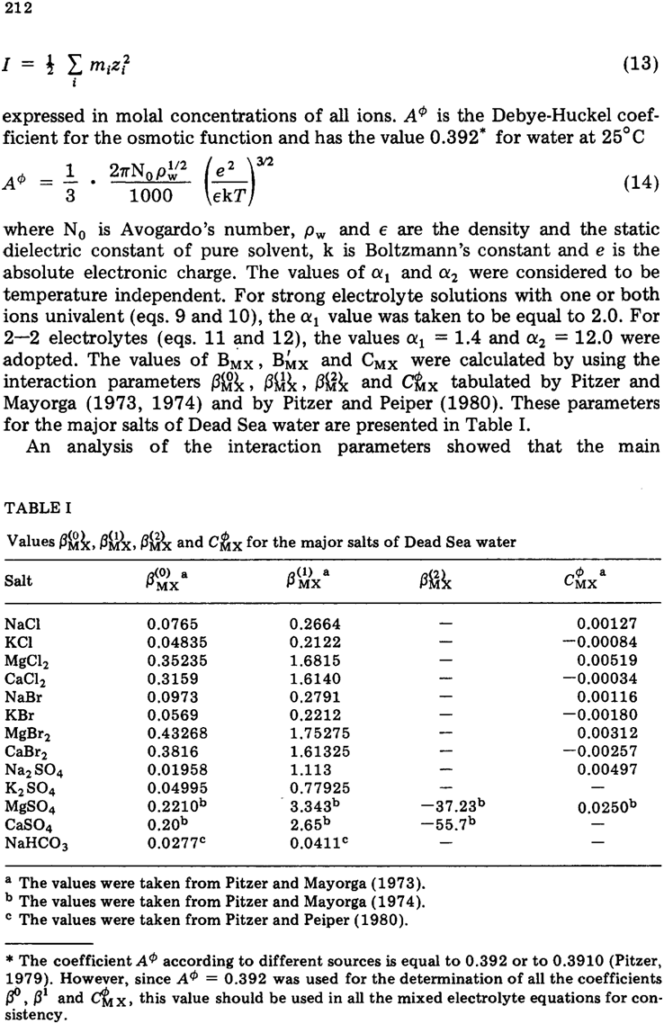
The chemical potentials of seawater components, including both the major ions and solvents in simplified seawater, were calculated by Robinson and Wood (1972), Leyendekkers (1973) and Whitfield (1973, 1974, 1975a) using a specific ionic interaction model. The calculated values were in reasonable agreement with the thermodynamic data available for seawater. The system of equations developed by Pitzer (1973, 1975, 1979), Pitzer and Kim (1974), Pitzer and Mayorga (1973, 1974), Dowries and Pitzer (1976), Pitzer and Silvester (1978), Pitzer et al. (1978) and Pitzer and Peiper (1980) for conventional single-ion activity coefficients makes it possible to extend the specific ionic interaction model over a range of solution compositions, temperatures and pressures. The aim of the present paper is to extend the Pitzer system of equations to the major components of Dead Sea water with an ionic strength approximately ten times that of ordinary seawater. The possibility of using these equations in concentrated solutions was demonstrated by Pitzer and Mayorga (1973) for single electrolytes and by Pitzer and Kim {1974) for mixed aqueous electrolytes. Harvie and Weare (1980) showed that a free energy model defined by binary- and ternary-system data can accurately predict mineral solubilities in brines with an ionic strength of 20 units of morality.