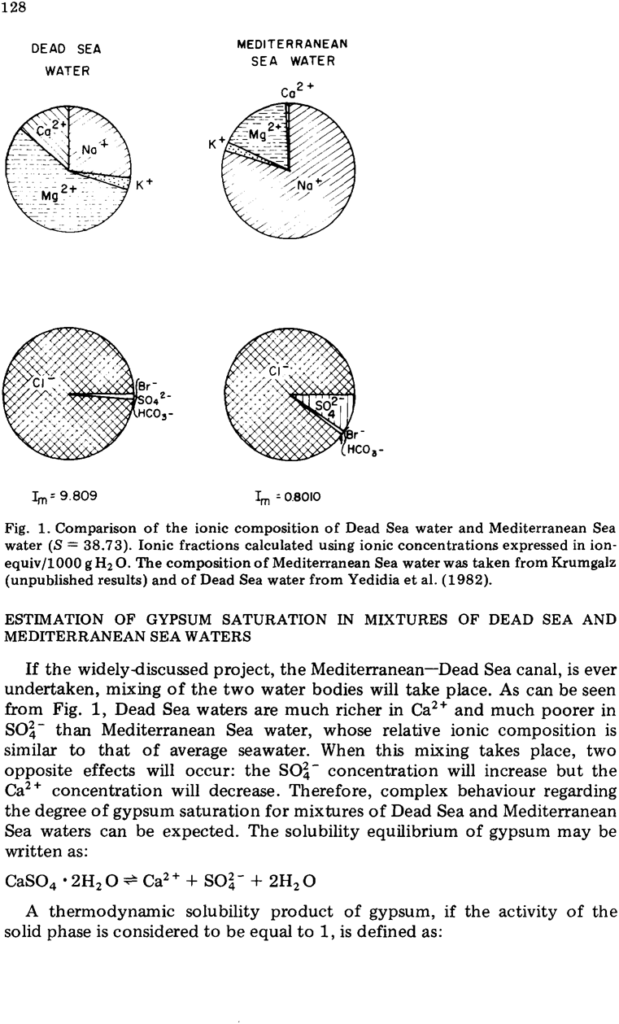
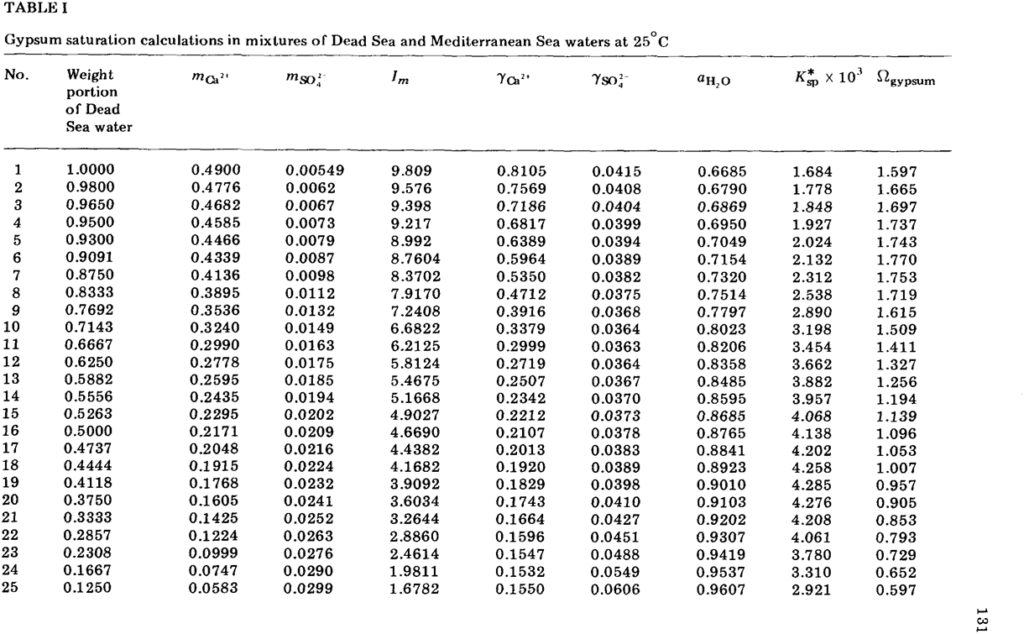
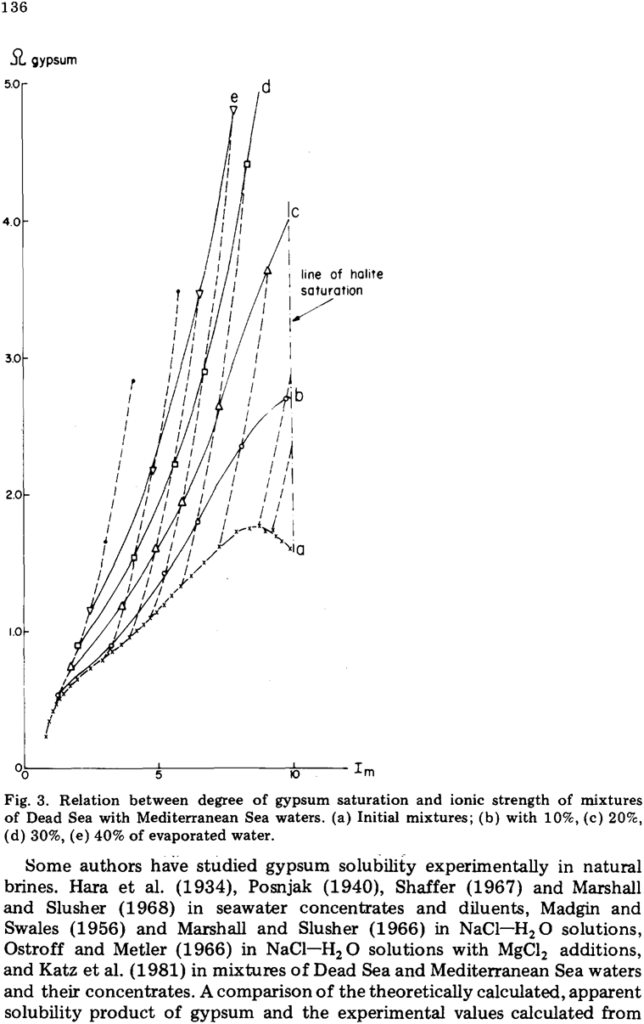
ABSTRACT
Krumgalz, B.S. and Millero, F.J., 1983. Physico-chemical study of Dead Sea waters. III. On gypsum saturation in Dead Sea waters and their mixtures with Mediterranean Sea water. Mar. Chem., 13: 127–139.
Gypsum solubility in mixtures of Dead Sea and Mediterranean Sea waters at different ratios and in their concentrates obtained by evaporation simulation have been calculated using the Pitzer model as formulated by Harvie and Weare (1980). The results obtained are compared with available experimental gypsum solubility data in different natural brines, sea water, seawater concentrates and NaC1 solutions. The agreement between theoretically calculated and experimental solubility values is fairly good and is within the range of scatter of the individual solubility measurements.
INTRODUCTION
The problem of gypsum (CaSO4″2H20) solubility in the Dead Sea has been considered recently by several research groups (e.g., Katz et al,, 1981; Krumgalz and Millero, 1982a). Both of these groups conclude that the Dead Sea is oversaturated with respect to gypsum. Earlier, Neev and Emery (1967) had shown that one of the sediment compounds in the Dead Sea is gypsum, which has been precipitated from Dead Sea waters during the last 100 years, perhaps longer. These authors observed that suspended material
in water samples consisted chiefly of gypsum and aragonite. However, in a recent study, Y. Levy {personal communication, 1981) did not find significant amounts of gypsum in suspended matter samples taken at different depths of the Dead Sea. This means that gypsum precipitation in the Dead Sea under present conditions is limited by some kinetic factors.