Abstract
Scientific exploration of the algal and cyanobacterial flora of the Dead Sea and its surroundings started in the 1930s. The unicellular green alga Dunaliella parva is the sole primary producer in the water column of the Dead Sea. The dynamics of the Dunaliella population and the interrelationships between the alga and the physical and chemical parameters in the lake are now well understood. Although cyanobacteria have occasionally been encountered in the Dead Sea as well, they are not known to contribute significantly to the microbial activities in the lake. Dense growth of cyanobacteria is found in various freshwater and saline, cold and warm springs in the Dead Sea area. Abundant cyanobacterial communities develop in the hot (up to 63 ºC) freshwater springs of Zerka Ma’in and Zara near the eastern shore of the lake. We here report data on the diversity of these communities, based both on microscopic observations and on molecular phylogeny techniques, and on their content of UV-absorbing pigments (mycosporine-like amino acids, scytonemin), which is of interest in view of the lowered levels of UV light reaching the Dead Sea shores.
Keywords: Dunaliella, cyanobacteria, Dead Sea, Israel, Jordan, hot springs
Introduction
The two quotations above give a depressing picture of the plant life in the Dead Sea and its surroundings, the lake itself being “weedless” with nothing but “weeds” around its borders. However, an examination of the world of microalgae and cyanobacteria inhabiting the lake and its surrounding area shows many interesting aspects that deserve in-depth study. The year 1936 was an important landmark in the exploration of the Dead Sea area for the presence of algal and cyanobacterial life. In that year, Benjamin Elazari Volcani (Wilkansky) (1915–1999), then a Ph.D. student at the Hebrew University of Jerusalem, published a short paper entitled “Life in the Dead Sea”, in which he first mentioned the occurrence of Dunaliella in the water column of the lake. In samples collected 3-4 km from the mouth of the Jordan River, “microscopic examination of a hanging drop of the water revealed the presence of a living phytoflagellate, 13µ long, believed to be either a Chlamydomonas or a Dunaliella” (Wilkansky,
1936) (Fig. 1, upper panels). In the spring of the same year, Tcharna Rayss (1890–1965), a professor of botany at the Hebrew University, joined by the famous French phycologist Abbé Pierre Frémy (1880–1944) and a group of students from Jerusalem, made a sampling trip to the warm springs of Zara—the ancient Kallirrhoe. A
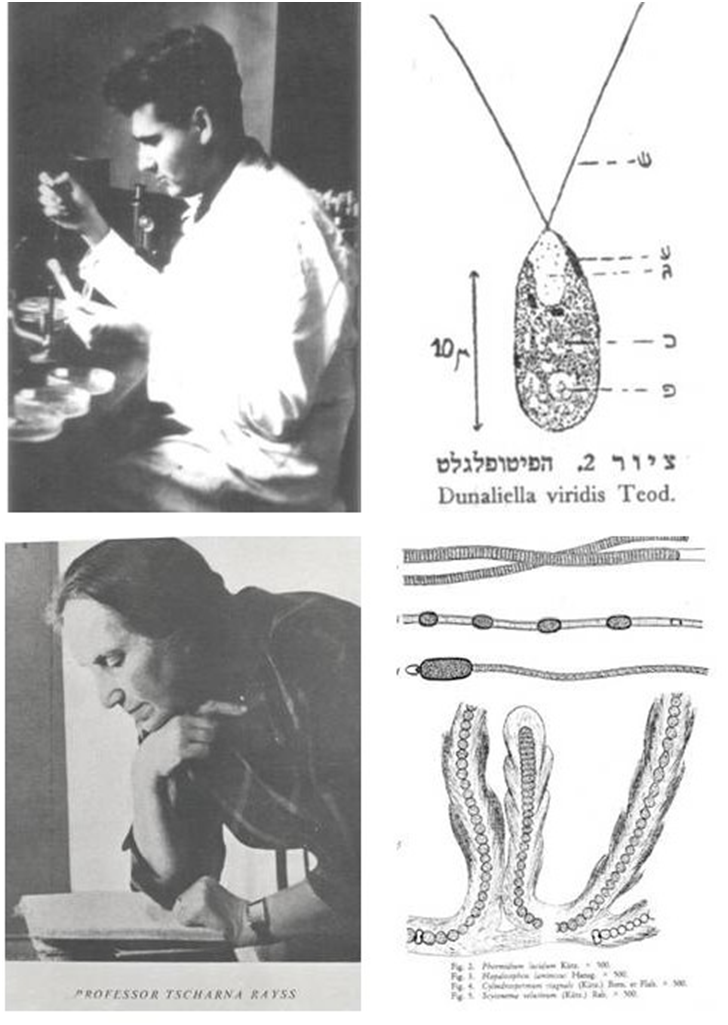
detailed report on the cyanobacterial communities found during the survey of the springs was published in 1938 in Volume 1, issue 1 of the Palestine Journal of Botany—Jerusalem Series, now the Israel Journal of Plant Sciences (Frémy and Rayss, 1938) (Fig. 1, lower panels). We here present a short review of the earlier published work on the algal and cyanobacterial communities of the Dead Sea and its surrounding springs, and include novel information from our recent surveys in the area to provide an updated overview of our current understanding of the microalgal and cyanobacterial diversity in and around the Dead Sea.
DYNAMICS OF DUNALIELLA AND OTHER PHOTOTROPHIC MICROORGANISMS IN THE DEAD SEA WATER COLUMN
The unicellular green alga Dunaliella is a common inhabitant of the Dead Sea. Since it was first reported
there by Volcani in the 1930s (Elazari-Volcani, 1940b; Volcani, 1944; Wilkansky, 1936), its presence was con
firmed in the early 1960s (Kaplan and Friedmann, 1970) and during blooms in 1980–1982 (Oren and Shilo, 1982) and 1992-1993 (Oren et al., 1995). Volcani identified the species as Dunaliella viridis. Nevo and Wasser (2000) named it Dunaliella parva Lerche, stating Dunaliella viridis Teod. sense Butcher 1959 as a synonym. The first attempts to count Dunaliella cells in the Dead Sea water column were made in 1964. Very high population densities of up to 40,000 cells/ml were encountered in surface waters (sampling date not stated). At 50 m depth, algal numbers were lower by two orders of magnitude, and no Dunaliella cells were detected in a sample collected from 100 m depth (Kaplan and Friedmann, 1970). A systematic monitoring program to follow the population densities of Dunaliella in the lake was initiated in 1980, and from that year onwards we have a good understanding of the occurrence of the alga in the lake (Oren and Shilo, 1982; Oren et al., 1995, 2005; Oren and Ben-Yosef, 1997; Oren, 2000). During the period of study, massive algal blooms developed twice in the surface waters of the Dead Sea: in 1980 (up
to 8,800 cells/ml) and in 1992 (up to 15,000 cells/ml and possibly even higher). These two bloom events occurred following exceptionally rainy winters in which unusually large amounts of freshwater caused a dilution of the upper 5–10 m of the water column, initiating meromictic conditions in the lake (1980–1982 and 1992–1995). In both cases, the algae were found all over the lake, with little spatial heterogeneity during the height of the blooms. No algae were observed the water column during the monomictic periods (1983–1991 and from 1996 until 2007), when this manuscript was finalized.The field observations can be understood on the basis of laboratory and outdoor mesocosm simulations that have shown that for a Dunaliella bloom to develop in the lake two factors must be fulfilled: (1) A significant (10% at least) dilution of the upper water layers is a prerequisite for an algal bloom in the lake, as undiluted Dead Sea water is too saline to support growth of Dunaliella. (2) Phosphate, which is the limiting nutrient, must be available (Oren and Shilo, 1985; Oren et al., 2004, 2005). Such simulation experiments are highly relevant in view of the planning of a Red Sea-Dead Sea water carrier (Oren et al., 2004). During the prolonged periods in which no algae can be observed in the water column, Dunaliella probably survives in the sediments as thick-walled resting stages (zygotes), which were actually observed to be formed during the decline of the 1992 bloom (Oren et al., 1995). This theory is consistent with the observation that the 1992 bloom started in the shallow areas all around the lake where the resting cells became exposed to the lowered salinity and spread afterward over the entire lake surface (Oren and Ben-Yosef, 1997).
Dunaliella is the only phototroph shown to grow and be active in the hypersaline waters of the Dead Sea.
There are a number of reports of sightings of other algae and of cyanobacteria in the lake (Vinogradova et al., 1996b; Dor, 1998; Nevo and Wasser, 2000), but most of the species mentioned are not known to be halophilic or highly halotolerant. To what extent they had developed in the Dead Sea should therefore be doubted. Bernard (1957) reported abundance of the coccolithophorid marine alga Coccolithus and the marine dinoflagellate Exuviella in Dead Sea water collected at the western shore, with densities up to 788 and 5,510 cells/ml, respectively.The true source of these algae can no longer be ascertained. Elazari-Volcani (1940b) published a short paper on “algae in the bed of the Dead Sea”, based on an examination of a sediment sample allegedly collected from a depth of 350 m (a value in error, being 15 m more than
the maximum depth of the lake at the time) from a station 8 km west of the east coast and “23 km west-southwest of the mouth of the Jordan” (apparently another error, as such a station would be located 10 km inland and 23 km from the eastern shore). In this and other Dead Sea sediment samples, Volcani found many algal species, including members of the Chlorophyceae (species of Scenedesmus, Pediastrum, Ulothrix); pennate and centric diatoms (genera Melosira, Navicula, Gomphonema, Cymbella, Pinnularia, Eunotia, Synedra); and unicellular and filamentous cyanobacteria (genera Aphanocapsa, Aphanothece, Oscillatoria, Phormidium, Plectonema) (Elazari-Volcani, 1940a,b). Volcani succeeded in culturing several cyanobacteria from these samples: Aphanocapsa, Microcystis (?), Phormidium
(in 18% salt medium), and Nostoc sp. (in 0–12% salt). A 0.5% salt medium yielded Chlorella sp., and two cultures with 12 and 15% salt yielded an unidentified small green flagellate (2–3 ´ 4–6 µm) with a single flagellum. The organism could grow over the whole range of salt concentrations between 1 and 27% (Elazari-Volcani, 1940a,b; Volcani, 1944). The finding of unicellular cyanobacteria such as Aphanothece and Aphanocapsa in the older Dead Sea studies is of special interest. They have also been found in hypersaline environments near the Dead Sea: the Hamei Mazor warm hypersaline sulfur springs (now
largely dried up) on the western shore of the lake (Oren, 1989) and experimental solar ponds (no longer operative) at the northern end of the lake near Bet Ha’Arava (Dor and Ehrlich, 1987). The too-high salinity and the special ionic composition of Dead Sea water prevents their active growth in the Dead Sea, but dense masses of Cyanothece/Aphanothece-like cells embedded in polysaccharide slime were observed to develop in experimental mesocosms in which Dead Sea water was mixed with an equal volume of water from the Red Sea (Oren et al., 2005).
ALGAE AND CYANOBACTERIA IN WARM AND COLD SPRINGS AROUND THE DEAD SEA
The surroundings of the Dead Sea are of special interest as a habitat for cyanobacteria and eukaryotic microalgae. The area is hot and arid, and therefore development of cyanobacterial desert crusts can be expected. Moreover, there is a great variety of springs with highly diverse chemical and physical properties: from cold to hot, and from freshwater to hypersaline, some having a high content of sulfide as well. As a result the area has attracted the attention of algal taxonomists who have performed surveys and prepared inventories of species found at the different sites. Information about the cyanobacterial species detected around the Dead Sea is especially abundant thanks to extensive studies by a number of experts over the past 70 years (Frémy and Rayss, 1938; Rayss, 1944; Dor and Ehrlich, 1987; Dor and Danin, 1996; Vinogradova et al., 1996a,b; Dor, 1998; Nevo and Wasser, 2000;). The monumental study by Ehrlich (1995) provides an excellent survey of the distribution of diatoms in the springs and other sites on the Israeli side of the Dead Sea shore. She listed at least 45 genera with at least 149 species of Bacillariophyta sighted in the area. A further discussion of the diatom communities in the Dead Sea springs is outside the scope of the present article, which will further center on cyanobacterial diversity. The very small numbers of species listed by Nevo and Wasser (2000) suggest that no extensive surveys for Chlorophyta and other classes of algae appear to have been made in the Dead Sea area. Based on the literature sources cited above, we can compile a long list of genera of cyanobacteria found around the Dead Sea. Species of Entophysalis, Schizothrix, Microcoleus, and Nostoc have been reported from the terrestrial areas. The freshwater springs and brackish water pools of Ein Fesha (Enot Zuqim) yielded no less than 29 species belonging to the genera Merismopedia, Chroococcus, Gloeocapsa, Gloeothece, Gomphosphaeria, Johannesbaptista, Oscillatoria, Schizothrix, Phormidium, and Nodularia. From the Ein Gedi area, Scytonema and Homoeothrix species were reported. Two species of Phormidium were identified in
the Ein Boqeq spring, and the warm springs of Hamei Zohar contained species of Gloeothece, Merismopedia, Chroococcus, Oscillatoria, Spirulina, Phormidium, Cylindrospermum, Scytonema, and Mastigocladus. Another rich source of cyanobacterial diversity was the area of the experimental solar ponds for the generation of electricity established at Bet Ha’Arava in the 1980s. These shallow ponds contained a bottom layer of Dead Sea water and an upper layer of brackish water, causing heliothermal heating of the lower water layers. At the time, rich communities of cyanobacteria developed along the salinity and temperature gradients in the ponds and in their vicinity, and these included species of Apha
notice, Aphanocapsa, Gomphosphaeria, Gloeocapsa, Chroococcus, Chlorogloea, Chroococcidiopsis, Phormidium, Spirulina, and Oscillatoria (Dor and Ehrlich, 1987 and other sources cited above). The operation of the system has since been discontinued. Beyond the taxonomic characterization of species,
there have been few ecophysiological studies on the cyanobacterial communities in the springs at the western side of the Dead Sea. In the 1980s, when there still were warm (39 ºC), hypersaline (around 170 g/l total dissolved salts) sulfur springs exposed near the shore south of En Gedi (Hamei Mazor), dominated by dense benthic growth of Oscillatoria or Phormidium-type filaments, it
was established that these cyanobacteria were capable of anoxygenic photosynthesis with sulfide as electron donor, driven by photosystem I, with the production of elemental sulfur, this in addition to oxygenic photosyn thesis with water as electron donor, driven jointly by photosystems II and I (Oren, 1989). This cyanobacterial community contained a high concentration of glycine betaine that served as the cells’ osmotic solute to withstand the high salinity of the environment (Oren et al., 1994). The exposed sulfur springs have since dried out, and the characteristic cyanobacterial growth has disappeared.The eastern shore of the Dead Sea presents us with two extremely interesting environments for the study of
cyanobacteria: the freshwater thermal springs of Zara near the lake’s shore and the even warmer (up to 63 ºC) springs in Wadi Zerka Ma’in, 4–5 km inland (Fig. 2). The cyanobacterial communities of these Jordanian hot spring areas have been the subject of our recent studies.
The cyanobacterial communities of the thermal springs of Zara and Zerka Ma’in
As mentioned at the beginning of this paper, the Zara hot springs (the ancient Kallirrhoe) (Donner, 1963) had been sampled and examined for algal life in 1936. The temperature of the water at the sampling sites varied between 35 and 40 ºC. Seventeen species of cyanobac
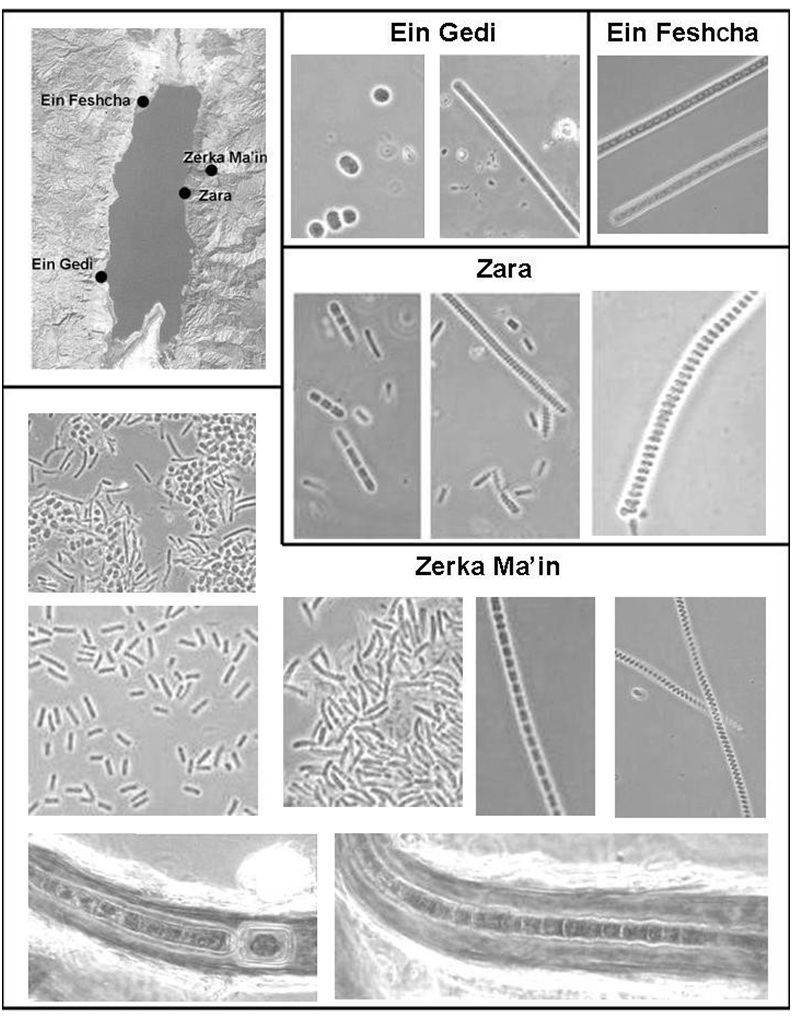
teria were identified, belonging to the unicellular genera Aphanocapsa, Merismopedia, Chroococcus, and Gloeothece and the filamentous genera Phormidium, Oscillatoria, Spirulina, Cylindrospermum, Hapalosiphon, and Scytonema (Frémy and Rayss, 1938; Fig. 1). A list published later by Rayss (1944) also mentions Aulosira thermalis. A few diatoms were present as well, but no attempt was made to further identify them.The presence of green-colored cyanobacterial mats in the freshwater hot springs in Wadi Zerka Ma’in was already mentioned by the first explorer who reached the area, Ulrich Jasper Seetzen, who visited the site in 1807. He noted that “In dem Wasser wuchs eine grüne schleimige Conferve” [In the water grew a green slimy microscopic alga] (Seetzen, 1854). More observations of the biological phenomena in the hot spring water were made by the German geologist Max Blancken horn, who surveyed the area in 1908. Blanckenhorn noted that where the water was particularly hot, blue green Cyanophyceae dominated. He found the bottom of the stream and the submerged rocks covered by green algal mats. Not being a specialist on algae, he rightly commented that “an algologist can find here, as well as in the other hot sulfur springs of Palestine, a wonderful area for observations and collection” (Blanckenhorn,1912; translation A.O.).Our joint Jordanian–Israeli studies of the cyanobacterial communities of the Zerka Ma’in springs were initiated in 2005 by the Bridging the Rift Foundation, and
the first results have recently been published (Ionescuet al., 2007, submitted). Conspicuous types include
unicellular Thermosynechococcus and Gloeocapsa, and Spirulina-like filaments. Spirulina labyrinthiformis was earlier reported as the dominant organism in material collected by A. Aaronson from a 52 ºC spring of the Zerka Ma’in (Rayss, 1944; Vinogradova et al., 1996b). Several representative types of Thermosynechococcus, Gloeocapsa, and Mastigocladus/Fischerella have been isolated and cultured. Amplification of cyanobacterial 16S rRNA genes from DNA extracted from the mats showed a high diversity of Thermosynechococcus (Ionescu et al., submitted). Low concentrations of ammonium and nitrate in the water and the presence of heterocystous cyanobacteria indicated that biological
nitrogen fixation may be important in the spring ecosystem. Genes related to nifH of Fischerella, Phormidium, and Lyngbya spp. were amplified from the community DNA. Laboratory experiments with heterocystous isolates showed the occurrence of nitrogen fixation (acetylene reduction) at 52 ºC but not at 63 ºC. However, reverse transcription PCR with mRNA isolated from a 63 ºC site amplified a gene identical to a nifH gene found in a filamentous cyanobacterial culture obtained from the site. Moreover, low but significant rates of acetylene reduction were measured during in situ incubations up to the highest temperatures in the springs. It is thus suggested that nitrogen fixation may occur in the cyanobacterial community of the Zerka Ma’in hot springs at temperatures significantly higher than the thus far recognized upper-temperature boundary for filamentous cyanobacterial nitrogen fixation of about 55 ºC (Ionescu et al., submitted).
Microscopical and molecular characterization of the cyanobacterial communities of the thermal springs of Zerka Ma’in and Zara
We have extended our evaluation of the morphological and molecular diversity of the Zerka Ma’in hot spring cyanobacteria. The spring sites around the Dead Sea explored during our surveys yielded a wide variety of morphological types, unicellular as well as filamentous. Figure 2 shows the most abundant types. Tightly wound Spirulina filaments were particularly abundant at the Zara site in outflow channels with water up to 44 ºC. At the hottest sites of the Zerka Ma’in springs, unicellular Thermosynechococcus-type cyanobacteria dominated. As the water cools down downstream towards the outflow channels, additional types of cyanobacteria start to appear, as pictured in Fig. 2. Cultures of Gloeocapsa and Mastigocladus/Fischerella were obtained from samples collected at different places in the Zerka Ma’in area. Near some of the outflow channels of the hot springs of the Zerka Ma’in, large colonies of the branching heterocystous cyanobacterium Scytonema were found (Fig. 2, lower panels). These colonies
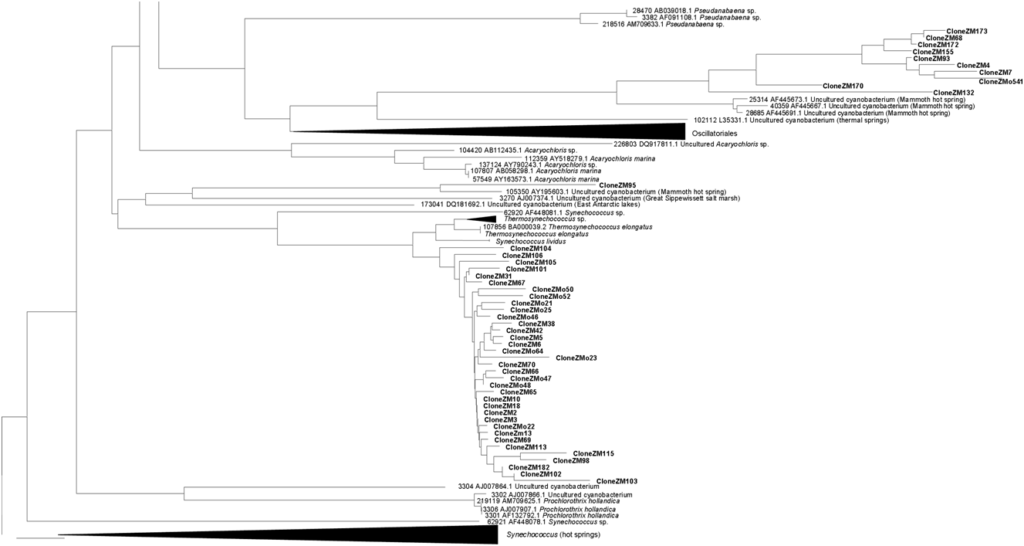
consist of dark-green to black material growing on the rocks, not in direct contact with the warm waters, but continuously sprayed by small droplets of water from the stream. Occurrence of Scytonema sp. was earlier reported from the Zara hot springs area (Frémy and Rayss, 1938). 16S rRNA gene sequences obtained from the environmental samples and from the cultures derived from them were aligned and plotted as a tree (Fig. 3). We have amplified 46 distinct sequences related to Ther mosynechococcus/Synechococcus from Zerka Ma’in (Fig. 3A). Unfortunately, no cultures of this type were
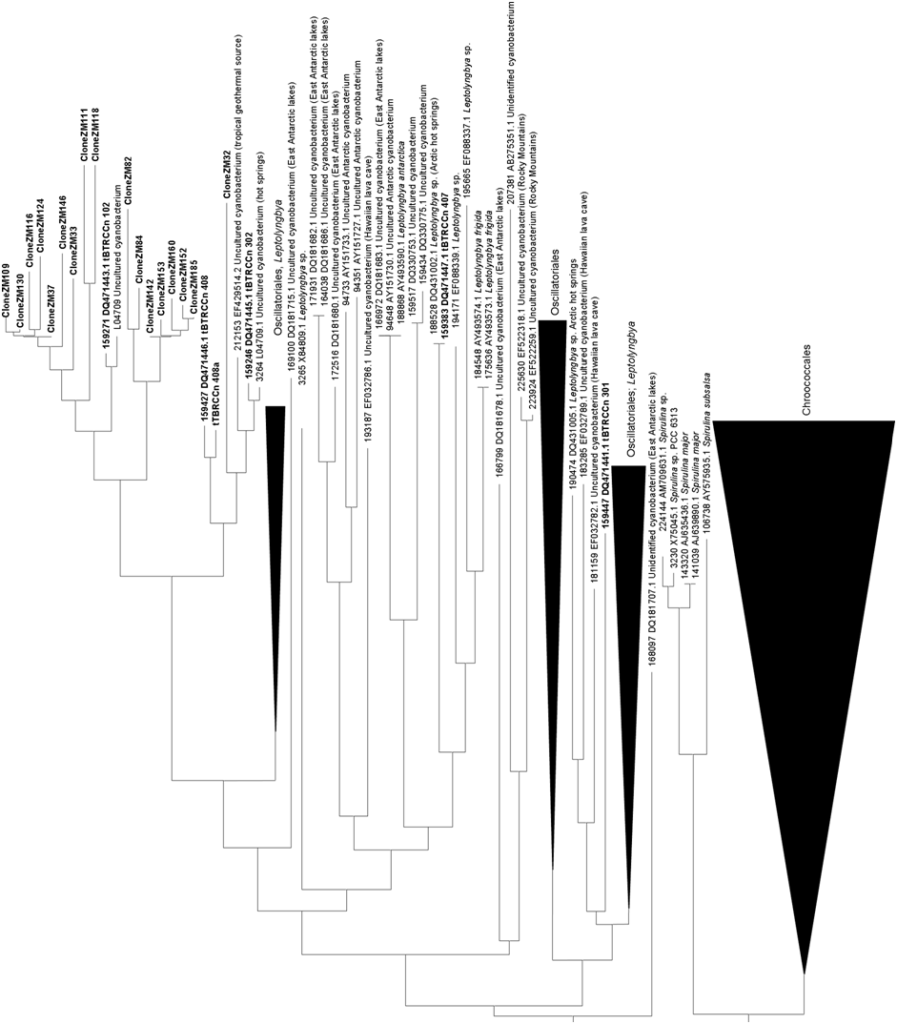
yet recovered from the springs. Filamentous non-heterocystous cyanobacteria are represented in the tree
(Fig. 3B) by 5 cultured strains and 17 environmental clones. In addition, we have isolated two cultures of
heterocystous cyanobacteria affiliated with the genera Fischerella and Mastigocladus from the springs
(Fig. 3C). No related sequences were yet detected among the environmental 16S rRNA gene fragments
cloned from the DNA isolated from the site. None of the

70 sequences was identical to any of the other cyanobacterial rRNA gene sequences found in the GenBank.
SURVEY OF UV-ABSORBING SUBSTANCES IN CYANOBACTERIAL COMMUNITIES IN SPRINGS AROUND THE DEAD SEA
The amount of ultraviolet solar radiation that reaches the surface of the earth is a function of the optical path length of the light. It is attenuated by atmospheric scattering by air molecules, water vapor, and aerosols, as well as by absorption by ozone, water, and carbon dioxide. The Dead Sea, being the lowest terrestrial place on earth, its current surface level being at 420 m below sea level, is therefore the place with the longest atmospheric optical path length. The amount of UV radiation penetrating to the area is therefore reduced. The levels of UV-B radiation at wavelengths above 310 nm at the Dead Sea (measured at 375 m below sea level) are about 12% lower than at an elevation of 315 m above sea level in a nearby area, and UV light of wavelengths shorter than 300 nm is much more efficiently attenuated (Kudish et al., 1997, 2003). The selective decrease in short-wavelength UV is considered one of the reasons for the success of the treatment of psoriasis and other skin diseases in health spas around the Dead Sea.
Organisms exposed to full sunlight have to protect themselves against the harmful action of UV radiation.
One strategy used by cyanobacteria is the accumulation of “sunscreen compounds” that absorb the harmful radiation. Two classes of such compounds have been identified: the mycosporine-like amino acids and scytonemin. Mycosporines and mycosporine-like amino acids (MAAs) are small (generally <400 Da) water-soluble molecules with maximum absorbance between 310 and 365 nm. They are accumulated by a wide range of microorganisms, prokaryotic (cyanobacteria) as well as eukaryotic (microalgae, yeasts, and fungi). MAAs are composed of either an aminocyclohexenone or an ami nocyclohexeneimine ring, carrying nitrogen or imino alcohol substituents (Bandaranayake, 1998; Sinha et al., 1998; Schick and Dunlap, 2002). Their biosynthesis is probably based on the shikimate pathway for the synthesis of aromatic amino acids. Thus far, the chemical structures of over 30 different MAAs have been elucidated.
In recent years evidence has accumulated that MAAs have additional functions: they may serve as antioxidant molecules scavenging toxic oxygen radicals, they can be

Fig. 4. Absorption spectra of pigment extracts of cyanobacterial material collected from the Zerka Ma’in thermal springs area. (A): a sample collected on November 16, 2006 from an outflow channel, GPS coordinates 8092506-4165670, water temperature 52 ºC), extracted in methanol–acetone 1:1 (v/v); (B): the same sample extracted in 20% methanol; (C): material from a nearby colony of Scytonema, extracted in acetone.
accumulated as compatible solutes following salt stress, their formation is induced by desiccation or by thermal stress in certain organisms, they have been suggested to function as an accessory light-harvesting pigment in photosynthesis or as an intracellular nitrogen reservoir, and they are involved in fungal reproduction. Thus, they may be considered as multipurpose secondary metabolites (Oren and Gunde-Cimerman, 2007). MAAs are found in a variety of cyanobacteria, unicellular as well as filamentous (Castenholz and Garcia-Pichel, 2000; Garcia-Pichel and Castenholz, 1993). They sometimes occur in high concentrations inside the cells: MAAs can accumulate to up to 0.8% of the cell dry weight in a Gloeocapsa (Garcia-Pichel and Castenholz, 1993), and in hypersaline environments even higher values have been reported in the literature (Oren, 1997).We have tested the presence of MAAs in material from the cyanobacterial communities collected on November 16–17, 2006 and on June 3, 2007 from the hot
springs of Zerka Ma’in (elevation approximately 250 m below sea level) and Zara (400 m below sea level), and the cold springs of Ein Fesha and the outflow channel of the warm hypersaline sulfur springs of Ein Gedi (400 m below sea level). The types of cyanobacteria encountered at the different sites are illustrated in Fig. 2. Samples were extracted overnight at 4 ºC in the dark with methanol–acetone 1:1 (v/v) (to extract UV-absorbing pigments as well as chlorophyll a, carotenoids, and scytonemin) or with 20% methanol to selectively extract MAAs without releasing the other pigments. Extracts were cleared by centrifugation, and their absorption spectra were recorded against the respective solvents in a Hewlett-Packard model 8452A diode array spectrophotometer. MAAs were absent or nearly absent from all samples collected at the highest temperatures (59–63 ºC). In most samples collected at 52 ºC and below, an absorption peak was found at 334 nm, which can be attributed to MAAs (Fig. 4A,B). This peak was in variably very small in comparison to the absorbance in the visible range of the spectrum due to cyanobacterial chlorophyll a and carotenoid pigments. Whether one or more types of MAAs were present in the samples, and whether all samples contained the same MAA or MAAs, could not be ascertained by the methods used. We did
not find signs of the presence of MAA compounds that absorb at longer wavelengths such as palythene (maximum absorbance at 360 nm) and related compounds. The summer samples did not contain higher MAA levels than those collected in the winter season. MAAs thus do not contribute much to the protection of the cells against UV radiation. The highest MAA concentrations, still very low compared to MAA-containing cyanobacterial communities found elsewhere, were encountered in the benthic community of the outlet of the hypersaline springs of Ein Gedi in November 2006, the MAA absorbance at 334 nm being about half the absorbance of chlorophyll a at 665 nm. In June 2007 this site had dried
up, so no sample was collected then.To test whether exposure to full sunlight at a higher elevation may induce the formation of MAAs, samples immersed in water from the respective sampling sites were incubated in open Petri dishes on the roof of the Institute of Life Sciences, the Hebrew University of
Jerusalem (elevation about 780 m above sea level) for 10 hours (7 a.m.–5 p.m.) under a clear sky, at a mid-day temperature of 18 ºC (November 2006) or 35 ºC (June 2007). Cells were then harvested, and extracted with methanol–acetone, and the absorption spectra were recorded. No significant increase in MAA content relative to the chlorophyll a peak at 665 nm was observed in any of the samples.
The results of our survey of the occurrence of MAAs in the springs around the Dead Sea can be summarized as follows:
1. MAAs are absent or nearly absent in the springs of
the highest temperatures.
2. In the lower temperature waters (52 ºC and below) MAAs were encountered (absorption maximum
around 334 nm), but always in low concentrations compared to other environments in which the occurrence of MAA sunscreen pigments has been documented.
3. Somewhat higher MAA concentrations were found in the halophilic cyanobacterial community at Ein
Gedi at the lowest elevation.
4. A 10-hour exposure of samples to full sunlight at an elevation of +780 m (albeit at suboptimal tem
peratures) did not induce synthesis of more MAA to compensate for the increased solar UV radiation.
The filaments of Scytonema, found in dense black-gray colonies near the outflow channels of some of
the Zerka Ma’in hot springs, are surrounded by a thick sheath (Fig. 2, lower panels) with the characteristic
the brown color of scytonemin. Scytonemin is a dimeric in dole alkaloid. It is probably synthesized from aromatic amino acid residues. It has an absorption maximum at 384 nm (Castenholz and Garcia-Pichel, 2000). Ab sorption spectra of extracts of this material in acetone or in methanol–acetone 1:1 showed the characteristic absorption peak of scytonemin, greatly exceeding the height of the chlorophyll absorption peak (Fig. 4C). Scytonemin has a broad absorption band, so that protection is provided against UV light of wavelengths shorter than the 384 absorption maximum as well, and the very
high concentrations at which this pigment is present in the sheaths surrounding the cells can therefore provide efficient protection against UV-induced cell damage. However, literature data suggest that the scytonemin content of those cyanobacteria that produce the pigment is poorly correlated with their actual exposure to UV light, and may be rather correlated with the occurrence of periods of restricted metabolism: the large investments required for effective use of scytonemin may only pay off when exposure is linked to periods of metabolic inactivity (Castenholz and Garcia-Pichel, 2000; Pente
cost, 1993). Scytonemin has never been reported to occur in truly thermophilic cyanobacteria. A survey of the hot springs in Yellowstone National Park, USA (elevation above 2000 m above sea level, so that ambient UV irradiance is high) showed UV screening pigments such as scytonemin to be absent above 55 ºC, the upper limit for growth of sheathed species such as Pleurocapsa and Calothrix (Wickstrom and Castenholz, 1978).
CONCLUDING REMARKS
The first survey of cyanobacterial diversity in the Dead Sea area (Frémy and Rayss, 1938) was published in
the first issue of the Palestine Journal of Botany—Jerusalem Series later renamed the Israel Journal of
Botany, now the Israel Journal of Plant Sciences. The current article, 70 years after the description of the
cyanobacterial diversity in the Zara Springs, published in the same journal, shows that our understanding of the algal and cyanobacterial diversity in the Dead Sea and its surroundings has increased considerably. We now have sufficient information about the occurrence of Dunaliella in the lake to be able to forecast under which conditions massive blooms of the alga can be expected to develop in the future. This is not only an exercise of academic interest, but the in-depth understanding of the biology of Dunaliella is highly important when trying to predict the implications of a Red Sea-Dead Sea or a Mediterranean-Dead Sea water carrier on the Dead Sea ecosystem (Oren et al., 2004, 2005). The opening of the border between Israel and Jordan since the peace treaty between the countries was signed in 1994 made both shores of the Dead Sea accessible to scientists from both countries. The joint Jordanian-Israeli surveys of the hot springs of Wadi Zerka Ma’in and of Zara, initiated in 2005 under the aegis of the Bridging
the Rift Foundation, enabled the renewed exploration of these sites using modern scientific methods, and have shown that the Jordanian hot springs are among the most interesting sites for the study of the diversity and physiology of cyanobacteria in thermal environments. It may be expected that the ongoing research at the sites will add new insights into our understanding of cyanobacterial life at high temperatures.
ACKNOWLEDGMENTS
We thank the Bridging the Rift Foundation for enabling the survey of the Jordanian hot springs and for financial support. We further thank Dr. Reuven Ortal of the Israel Nature and National Parks Authority for permission to collect samples at the Ein Fesha nature reserve, and Mr.Boaz Ron for allowing access to the Ein Gedi spa site.








