ABSTRACT
The present review is dedicated to the world’s lithium resources and application areas of
lithium and its compounds in everyday life and technology. Lithium is the lightest representative in the group of alkali metals. In its geochemical properties lithium refers to a group of lithophilic elements. Lithium is found in more than 150 minerals, although only 28 lithium minerals per se are known. Many of them are extremely rare. The main lithium minerals are the following: amblygonite, lepidolite, petalite, spodumene, zinnwaldite and Jadar. A review of the world’s largest lithium deposits made the analysis of its global production and reserves. Deposits of lithium are known in Chile, Salar de Uyuni in Bolivia, United States, Argentina, Congo, Lake Chabyer in China, Brazil, Serbia, and Australia. The estimates of
reserves were made on the basis of information received from government and industry sources. Separately was an analysis of the resources and reserves of lithium in the associated
petroleum waters of Russia. It also shows that a large source of lithium is the Dead Sea in Israel. Due to the market development of lithium power sources, the most promising lithium resource is secondary resources. The necessity of creation of processes for recycling of spent lithium power sources. In connection with the rapid growth in lithium consumption, it is very urgent task of extracting lithium, and receive it in the form of salts, and metal. Have been described, modern sorption methods of lithium extraction from poor on the composition of natural waters and brines with the use of inorganic ion exchangers highly selective to lithium. We present the results of their tests on real natural brines. Particular attention is given to the review of lithium and its compounds application areas. The most important and rapidly growing area is lithium power sources. Next in importance and volume of consumption lithium is glass and ceramics. Also, large application areas of lithium are lubricants, regeneration of oxygen in autonomous life support systems, production of polymeric materials and catalysts in chemical technology, metallurgy, pharmaceuticals, medicine. In recent years, a number of promising new application areas of lithium and its compounds: are hydrogen energy, electronics and nonlinear optics, nuclear power, and used as rocket fuel. At the end of the article, it provided an overview
of prices and demand for lithium and its compounds.
Keywords: Lithium, Lithium geochemistry, Lithium application, Extraction methods of Lithium,
Lithium geochemistry
Fields. world production and reserves
- Salar de Uyuni in Bolivia
- Chile
- United States of America
- China, Lake Chabyer
- Russia
- Israel, Dead Sea
- Secondary lithium resources
The use of lithium isotopes
Extraction of lithium, and its preparation in the form of salts and metals
The use of lithium and its compounds
- Lithium power sources
- Glass and Ceramics
- Lubricants
- Regeneration of oxygen in the autonomous life support systems
- Polymer materials and chemical technology
- Metallurgy
- Pharmaceuticals, medicine and biological importance of lithium
- Prospective application field of lithium and its compounds –
Hydrogen energetics – – –
Electronics and nonlinear optics
Nuclear energy
Rocket fuel - Other applications
Cost of lithium
Demand for lithium
References
LITHIUM GEOCHEMISTRY
Lithium is the lightest representative in the group of alkali metals. In its geochemical properties lithium refers to a group of lithophilic elements. The ions of these elements have large dimensions. These include the potassium, rubidium and cesium. The lithium content in the upper continental crust is 21 g/ton and in the seawater is 0.17 mg/l [1]. Lithium is found in more than 150 minerals, although only 28 lithium minerals per se are known. Many of them are extremely rare. The main lithium minerals are the following: amblygonite, lepidolite, petalite, spodumene, zinnwaldite and Jadar. The most common one is: lepidolite –
KLi1,5Al1,5 Si3AlO102, which belongs to a group of mica minerals, i.e., aluminosilicate having a layered structure, and the spodumene – LiAl[Si2O6], referring to a group of pyroxenes, i.e., chain silicates. In many cases, lithium does not form independent minerals and is isomorphic to replace potassium in wide-spread rock-forming minerals. Deposits of lithium are associated with rare metal granitic intrusions, which result in development of lithium-carrying pegmatites or hydrothermal complex deposits of other metals. Lithium also occurs in the underground thermal waters with extremely high concentrations of various trace elements. Other common type of lithium deposits are natural brines of some highly saline lakes.
FIELDS. WORLD PRODUCTION AND RESERVES
Deposits of lithium are known in Chile, Bolivia, United States, Argentina, Congo, China, Brazil,
Serbia, and Australia. The estimates of reserves were made on the basis of information received from
government and industry sources [3, 6] and are presented in Table 1. Data in terms of US domestic
production has not been published, and, in order to protect our own data, we evaluated them on the
basis of data published on reserves.
| Country | Production years | Reserves, ton | World Resources | ||
| 2014 | 2015 | ton | % | ||
| Bolovia | 9 000 000 | 21.4 | |||
| Chili | 11 500 | 11 700 | 7 500 000 | 7 500 000 | 17.8 |
| USA | 1130 | 1130 | 38 000 | 6 700 000 | 15.9 |
| Argentina | 3 200 | 3 800 | 2 000 000 | 6 500 000 | 15.4 |
| China | 2 300 | 2 200 | 3 200 000 | 5 100 000 | 12.1 |
| Australia | 13 300 | 13 400 | 1 500 000 | 1 700 000 | 4.0 |
| Israel3 | 1 000 000 | 2.4 | |||
| Canada | 1 000 000 | 2.4 | |||
| Congo (Kinshasa) | 1 000 000 | 2.4 | |||
| Russia | 41 000000 | 2.4 | |||
| Serbia | 1 000 000 | 2.4 | |||
| Brazil | 160 | 160 | 48 000 | 180 000 | 0.4 |
| Mexico | 180 000 | 0.4 | |||
| Austria | 130 000 | 0.3 | |||
| Portugal | 300 | 300 | 60 000 | 60 000 | 0.1 |
| Zimbabwe | 900 | 900 | 23 000 | 23 000 | 0.1 |
| Totalinthe world2 | 32000 | 33000 | 14 400 000 | 342000000 | 100 |
1 – author’s estimates, official data are not available;
2 – approximated;
3 – calculated by the author;
4 – without taking into account underground brines.
Salar de Uyuni in Bolivia
Salar de Uyuni in Bolivia is the world’s largest deposit of lithium, which is also suitable for the
extraction of lithium chloride by a halurgical method. The Bolivian press reported that in this saline
lake contains about 100 million tons of lithium. However, this value is 10 times higher than the
estimates of American experts [2]. According to experts from the U.S. Geological Survey (USGS) the
aforementioned deposit contains 50 to 70% of the world’s lithium reserves [4].
Chile
Reserves of lithium ore In Chile takes second place among the industrialized capitalist and
developing countries (50% of proven reserves). Deposits (Salar de Atacama, Askotan, etc.) are
located in the Central Valley and are associated with mineralized water of salars mountainous undrained lakes. In the Salar de Atacama field, lithium resources in the “caliche” (porous gypsum
halite rocks soaked in brine) are estimated in excess of 3 million tons with 0.3% Li2O content.
United States of America
In the United States two companies operate in the fields of extracting and processing lithium raw
materials. One of these companies is Rockwood Lithium Inc., Kings Mountain, North Carolina, and
Lithium Corporation in Nevada that uses a salt lake in Fish Lake Valley.
Rockwood Lithium, Inc. is a world leader in the market for lithium compounds and one of the
largest manufacturers of lithium raw materials. The company is also the world’s leading supplier of
special metal compounds based on cesium, barium or zirconium. Access to raw materials is
fundamental to the economy and is vital to ensure supplies. Rockwood Lithium, Inc. is fully backward
integrated and has to use three independent resources of primary lithium.
Chilean resource in Salar de Atacama, which is the most attractive in the world, is in the activities
of Rockwood Lithium, Inc. since 1980. Since 1960’s, Rockwood Lithium, Inc. exploits one more
plants, which is located in Silver Peak, Nevada and operates on brine. In 2014, the company acquired
a stake of 49% from Talison Lithium in Australia with access to its spodumene resources. In addition,
Rockwood Lithium, Inc. is constantly working on innovative methods of operation to improve the
efficiency and sustainability of its resources [9].
Lithium Corporation is a young mining company that is focused on creating a shareholder value
through the discovery and development of deposits of lithium and other associated minerals.
Currently, Lithium Corporation studies two fully owned promising fields located in Nevada, USA,
and two fields located in British Columbia, Canada. In each of the Nevada fields the company
discovered an anomaly on the content of lithium in the brine. In its flagship mine, which is located in
Fish Lake Valley, in addition to lithium, the company found reserves of potassium and boron. in 2011,
near the surface of the lake, the company ran onto brines enriched in lithium and boron and containing
up to 140 mg/l of lithium. In addition to these highly anomalous values of the content of lithium and
boron, the deposit has an increased content of potassium which reaches 2500 mg/l. The company
undertook a drilling program that was conducted during the autumn of 2012. Significant works in this
direction are included into the plans of 2016 [10].
China, Lake Chabyer
A third unique source of lithium is Zabuye salt Lake – mountainous undrained highly-saline soda
lake in the county of Shigatse, Tibet Autonomous Region, China. Besides the enormous amount of
lithium carbonate, Lake Zabuye contains appreciable quantities of sodium tetraborate, Glauber’s salts,
and other alkali metal salts. The content of the chemical elements (g/l): Na+ – 160, Cl – 120, K+ – 60,
Br+ – 3, B – 3, Li+ – 1,2 ÷ 1,53, Rb+ – 0,25, Cs+ – 0 1, I- – 0,02; – 90, 𝐶𝐶3 2− – 20. The density of water is
1.4 g/cm3, pH 10. In 2008, Zabuye (Shenzhen) Lithium Trading Co., Ltd. produced 1556.5 tons of
lithium carbonate. The company plans to increase the capacity of its plant in the near future from
5,000 to 20,000 tons per year, assuming that the lake reserves are 8.300.000 tons of carbonate
(1.530.000 tons of lithium). However, many experts believe these numbers are overestimated [5].
In the 1984, a mineral known as Zabuyelite (lithium carbonate, Li2CO3) was discovered in the
area of this lake, however, only in 1987 it was proved that this mineral can be used for the production
of lithium in industrial quantities. In 1999, a company known as Zabuye (Shenzhen) Lithium Trading
Co., Ltd was founded here and started production of lithium, and in 2005 [5] this salt lake mine was
recognized as the largest source of lithium throughout China. In 2008, Zabuye (Shenzhen) Lithium
Trading Co., Ltd. had a staff of 50 employees. In 2008 the company’s plant on the shore of the lake
produced 1556.5 tons of lithium carbonate. In the near future, the company plans to increase the
capacity of its plant from 5000 to 20 000 tons per year [2], evaluating lake stocks as 8.3 million tons of carbonate (1.53 million tons of lithium). However, many experts believe these numbers are
exaggerated [5]. Tibet’s government is actively investing in the development of the lake. According to the
government estimation, in addition to 20,000 tons of lithium carbonate, the plant will be able to
produce annually 5,000 tons of lithium chloride, 500 tons of conventional lithium, 200 tons of ultra
pure lithium, and 520 tons of other lithium-containing substances.
Russia
In Russia more than 50% of lithium reserves are concentrated in rare metal fields of the
Murmansk region. However, in our opinion the data presented in Table 1 is too low; since this data
does not take into account the resources of lithium found in subsurface brines and associated
petroleum waters. Practically, in many regions of Russian Federation there are deposits of salt
groundwater with lithium having concentration of 5 to 50 mg/l.
Israel, Dead Sea
Saline Dead Sea basin is a part of one of the largest global zones of salt accumulation – Afro
Arabia, which is inside the Intercontinental rift (submeridional). The African-Arabian rift belt is
characterized by salt-bearing basins of different types of rift structures: inland rifts (Suez Graben,
separate branches of the East African System) of within shear zones (the Dead Sea) and inter
continental rifts (Red Sea). Saline Dead Sea basin began to form in the first stage of the Late Triassic
period, about 230 million years ago. The second stage of its formation took place in the Cenozoic era,
between Pliocene and Holocene, i.e., from 6.7 million to 10000 years ago. Accumulation of salts
continues also in the modern era. In addition, the structure of the salt basin of the Dead Sea is
complicated by hydrochloric dome tectonics. In geodynamic classification of sedimentary basins of
salt, it is characterized by the formation of the spreading rift geodynamic regime [24]. In this regard,
the Dead Sea is just a small external surface manifestation of giant salt deposits stretching from the
southern tip of the Arabian Peninsula to the Strait of Gibraltar. Hence, the water of the Dead Sea is a
product of dissolution of a small element of this salt bed that comes to the surface. Therefore, our
evaluations of lithium resources in the Dead Sea area are underestimated and relate only to the
territory directly near the Dead Sea.
The content of mineral substances in the Dead Sea water is up to 340-350 ‰, 300 ‰ in average
(for comparison, in the Mediterranean Sea – 40 ‰). The Dead Sea is one of the most saline lakes in
the world. The mineralogical composition of salts of the Dead Sea is significantly different from the
composition of the salts of other seas. It contains about 50.8% magnesium chloride, 14.4% calcium
chloride, 30.4% sodium chloride, and 4.4% of potassium chloride. It contains a relatively small
amount of sulfates, but relatively large amount of bromides.
In its borders the Dead Sea exists for about 15,000 years, and during this time a 100 meter thick
salt and mud sediment layer has accumulated at the lake’s bottom. The volume of water in the Dead
Sea is about 110 km3, and it contains about 50 billion tons of dissolved mineral substances. It contains
21 different minerals. The chemical composition of the Dead Sea is shown in Table 2.
We evaluated lithium resources in the Dead Sea on the basis of published data on reserves of
magnesium, potassium, and bromine [25], and the concentration of these elements in the water of the
Dead Sea (Table 2). The results are shown in Table 1.
| Components of the Dead Sea Water Composition | Concentration | |||||
| GeneralData | Nissenbaum, (1974) [26] | Nissenbaum,(1977) [27] | ||||
| (1), g/l | (2),mg/l | mg/l | mkg/l | mg/l | μg/l | |
| Na+ | 34.9 | 39158 | 39200 | |||
| K+ | 75.60 | 7956 | 7300 | |||
| Rb+ | 0.06 | |||||
| Ca2+ | 15.8 | 16900 | ||||
| Mg2+ | 41.96 | 45345 | 40700 | |||
| Cl– | 208.02 | 227545 | 212400 | |||
| Br– | 6.92 | 5360 | 5100 | |||
| SO42- | 0.54 | 500 | ||||
| CO32- | 0.24 | 200 | ||||
| Li+ | 20 | 17÷21 | ||||
| Sr2+ | 300 | 308÷330 | ||||
| Mn2+ | 4.0÷7.1 | 3.1÷8.0 | ||||
| Cu2+ | 300÷500 | 300÷500 | ||||
| Zn2+ | 500 | 500 | ||||
| Fe2+ | 10÷15 | 10÷15 | ||||
| Ni2+ | 20÷25 | |||||
| Co2+ | 8 | 8 | ||||
| Cd2+ | 8÷10 | 8-10 | ||||
| Pb2+ | 120÷300 | 120÷300 | ||||
| I- | 80÷120 | |||||
| U(VI) | 1.3÷2.5 | 1.5÷2.5 | ||||
| Salinity | 315 | 343202 | ||||
Secondary lithium resources
One aspect of the efficient use of the lithium resources is possibility of recycling of lithium
containing wastes. Historically, recovery of lithium from secondary resources was insignificant, but it
is constantly increasing due to the growth of consumption of lithium by lithium batteries. Thus,
reprocessing or use of secondary resources will become increasingly important over time. This trend
needs to be developed in the present time. As an example of such a development, we can refer to the
activity of Rockwood Lithium, Inc. in the USA.
This company recycles lithium metal and lithium-ion batteries already since 1992 at its plant in
British Columbia, Canada. In 2009, the US Department of Energy gaves the company a grant of $ 9.5
million for construction of the first US center for utilization of lithium-ion batteries for vehicles. The
company provides technology for recovery of lithium or cesium contained in the waste from different
industries, and manufactures products from these secondary sources. It is safe to predict that car
batteries from hybrid or electric vehicles with expired service-life, will in the near future become the
most important secondary raw material for the lithium industry. In order to be ready for this,
Rockwood Lithium, Inc. is now developing appropriate processes and began to build a pilot plant for
reprocessing lithium salts of EV batteries. The construction is nearing completion.
THE USE OF LITHIUM ISOTOPES
Natural lithium consists of two stable isotopes: 6Li (7,5%) and 7Li (92,5%); in some samples of
lithium the isotope ratio may be much disturbed as a result of natural or man-made isotope
fractionation.
6Li has a greater affinity with mercury than 7Li. This is the basis for the enrichment process
COLEX [7]. An alternative process is vacuum distillation that occurs at temperatures around 550°C.
Normally, separations of lithium isotopes are needed for military nuclear programs (Russia, USA,
China). In the USA, 7Li was produced only as a byproduct of a military nuclear program, namely, in
the process of lithium enrichment in the isotope 6Li. Currently, only Russia and China [8] have
functioning capacity for the separation of lithium.
In the United States, the 7Li is actively used in reactors PWR, analogues of Russian Water-Water
Energetic reactors. There are 100 nuclear reactors in operation, of which 65 have installed tank
reactors PWR. The water-chemistry mode with PWR units requires the use of lithium hydroxide,
enriched in the isotope 7Li. In addition, the lithium-enriched compounds are used in demineralizers for
cleaning the primary coolant circuit. The use of lithium enriched in the isotop 7Li, rather than natural
lithium, is necessary because isotope 6Li interacts with neutron by the reaction (n;α) to form tritium.
Previously it was thought that China and Russia have produced 7Li in an amount sufficient to
meet the current needs of the United States, which is unlikely to increase significantly in the
foreseeable future. However, supply volumes from China may be reduced due to the active
construction in the country of new nuclear units. In addition, China has plans to develop molten-salt
reactors, whose operations will require large amounts of 7Li. China has developed two types of
molten-salt reactors which may require large volumes of 7Li. For comparison, the annual demand of
all 65 US units with PWR in 7Li is approximately 300 kg. Furthermore, molten-salt reactors require
significantly higher concentration of lithium 7Li, namely – 99.995%. Currently, there is no 7Li of such
purity in China.
China has built a small laboratory, whose mission is to enrich the Chinese stocks of 7Li.
Functioning of this laboratory would inevitably lead to a decrease in volumes of the sales of 7Li from
Chinese side. Moreover, it is known that China has already started to acquire 7Li in Russia.
Unlike China, Russia has low need in domestic consumption of 7Li and can be a supplier. On the
Russian units corrosiveness of H3BO3 is reduced by using potassium hydroxide instead of lithium.
The auditors of US Government Accountability Office proposed three solutions to the problem of
potential risks of interruptions in the supply of 7Li [11].
First, the cheapest way is to create a national reserve of 7Li by increasing the volume of imports
and to clean the remaining reserves of lithium at the Factory Y-12 (1300 kg of lithium-7). The cost of
such an approach can be determined based on the current price of less than $10 000 for 1 kilogram of
lithium-7. However, buying should be done carefully so as not to cause a sharp rise in prices and not
to cause other damage to the market. The cost of cleaning of 7Li obtained from the military Factory Y
12 can be judged based on the known data stating that cleaning of the first 200 kg cost $ 3,000 per
kilogram. It is possible that cleaning of the remaining 1100 kg will have a higher rate per kilogram.
The second way is restoration of production of 7Li in the USA. According to the estimate by
specialists of the Factory Y-12, creation of manufacture capable of delivering only 200 kg of 7Li per
year will take five years and require investment of 10-12 million dollars.
Finally, the third way is to reduce the needs of PWR-containing blocks in 7Li. This can be
achieved at the expense of recovery of 7Li from demineralizators. Next reserve is to reduce a need
in 7Li, i.e., to switch to the use of potassium hydroxide as is customary in Russian VVERs. Industry
estimates the time for such transition as 10 years [11].
EXTRACTION OF LITHIUM, AND ITS PREPARATION IN THE FORM OF SALTS AND
METALS
Currently, to extract lithium from its natural minerals, they are decomposed by the action of
sulfuric acid (acid method), or sintered with CaO or CaCO3 (alkali method), or treated with potassium
sulfate K2SO4 (salt method), and then leached with water. When a lithium raw material is comprised
of natural brines, it is extracted by a halurgical method based on the difference in solubility’s of
sodium and lithium chlorides in concentrated solutions and in the presence of other metal salts. Brines
poor in lithium require the use of a sorption lithium extraction method with the use of selective
sorbents. Since the lithium-rich brines can be quickly exhausted, an agenda will be a question of
lithium extraction from relatively lithium-poor sources of raw materials.
In any case, a preprocessing produces a concentrate solution of lithium salts. The resulting
solution is used for recovery of poorly soluble lithium carbonate Li2CO3, which is then converted into
chloride LiCl. Melt electrolysis of lithium chloride is carried out in admixture with KCl or BaCl2
(these salts are used to lower the melting temperature of the electrolyte mixture):
2𝐿𝐿𝐶𝐿 → 2𝐿𝐿 + 𝐶𝐿2 ↑
Further, the resulting lithium metal is purified by vacuum distillation at a temperature of about
550°C.
Lithium can be leach out of the rocks, and the most common raw source of this element is natural
waters. The content of lithium in seawater averages 1.5 to 10-4 g/l. From a technological point of view
such concentration is low. Much more promising are some lake brines. The maximum known lithium
concentration of 56 mg/l is observed in chloride-type lake waters. Accordingly, lakes are considered
as real sources of a lithium raw material. For example, in the Great Salt Lake (Utah, USA), the total
lithium content reaches 4 million tons (based on LiCl) with lithium concentration of 20 mg/l and
higher.
It has been shown that a content of lithium in brines can be concentrated by natural evaporation.
Experiments made still in the USSR confirmed increasing the lithium concentration in the brine
during its evaporation from 2.1 to 32.5 mg/l, but the total mineralization of brine in this case was
increases from 280 to 582 g/l.
A study has been conducted regarding a possibility of extracting lithium from free petroleum
waters of Dagestan (in the area of South Sukhokumsk). A mixture of free saline waters had the
following composition (g/l): NaCl – 75,5; KCl – 15.8; CaCl2 – 20.3; MgCl2 – 2,20; SrCl2 – 1,22; and
LiCl – 0,52. It was planned to carry out complex processing of these raw materials. However, the
studies have ceased in the early 90s.
Schemes of the existing industrial complexes for processing lithium-containing brines are
oriented on Li-rich sources (with Li content in the range of 300 to 500 mg/l), and are based mainly on
evaporation and sequential crystallization of different compounds [18-19]. The sorption methods
appear to be most promising for the brines with poor content of rare elements [20-23]. Separation of
lithium from solutions in the form of carbonate is effective when its content in the solution is not less
than 20 to 25 g/l. For brines with the lithium concentration of 40 mg/l this corresponds to a
concentration degree of 500. On the other hand, ion exchange processes developed for the above
purpose provide not only a required degree of concentration but also specified production efficiency.
The problem of extracting lithium from salt brines is largely connected with their molar ratio of
ion Na+ to Li+, i.e., the value of Na/Li. Sodium is one of the elements which is the closest to lithium in
its chemical properties and which, as a rule, also has the highest content in natural waters. In some
classes of chloride brines the Na/Li ratio can reach 93000. Under such conditions conventional
methods based on the precipitation of sparingly soluble compounds of lithium, such as phosphates,
are not effective. Promising are only sorbents, which are highly selective to Li+ ions. Inorganic sorbents such as ICM-1 have been developed for the extraction of lithium from highly
mineralized natural brines. The basis of these sorbents is manganese dioxide. Under the effect of
thermal recrystallization the manganese dioxide acquires a structure in which the cation-exchange
positions strictly correspond to Li+ ions, while access of Na+ ions inside the crystal grains is limited
due to the “ion-sieve” effect. The sorption-desorption processes are accompanied only by H+ ↔ Li+
type exchange. Main features of the ICM-1 sorbent are partition coefficients for the H+ – Li+ ion pair,
which reach (1 ÷ 5)·104 ; the exchange capacity of 4 ÷ 5 mol Li+/kg (full); and 1,0 ÷ 2,0 mol Li+/kg
(working capacity in solutions of complex salt compositions); and the residual concentration of Li+
ions in the filtrate after sorption in the range of 0.1 to 0.3 mg/dm3.
The modified sorbent ISMA-1 was tested in the process of lithium extraction from the
underground solution of iodine-bromide production (Perm, Russia). The solution had the following
composition (g/dm3): NaCl – 189; CaCl2 – 56.05; MgCl2 – 14.9; KCl – 2.47; NaBr – 1.12; SrCl2 –
0.42; Na2B4O7 – 0.12; KI – 0.02; Li+ – 11·10-3, pH 8,2. Sorption was performed prior to breakthrough
of Li+ ions to the filtrate – 0.9 mg/dm3. Test results: sorption capacity was 17 g Li+/kg, the degree of
lithium extraction was 91.5%. The author took active part in the development of technology for these
and other similar sorbents, as well as in the test of their properties in case of real industrial and natural
solutions [12-16].
THE USE OF LITHIUM AND ITS COMPOUNDS
The scheme of lithium consumption has not changed much over the past few decades. However,
major changes have occurred in the proportions.
In recent years the consumption of lithium for the batteries has significantly increased. This is
because the market has grown for rechargeable lithium batteries to be used in portable electronic
devices, electric tools, electric vehicles, and applications to network storage circuits. Lithium minerals,
as before, are used worldwide directly as ore concentrates in the ceramic and glass industries. The
structure of the largest areas of use of lithium and its compounds in the world in 2011 and 2016 is
presented in Figure 1.
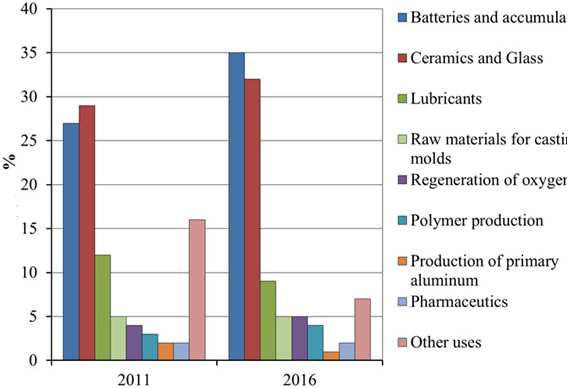
Figure 1. Assessment of the largest areas of use of lithium and its compounds in the world in 2011
and 2016. [3].
Lithium power sources
For many years, the first and most important application of lithium and its compounds was the
sphere of chemical current sources. Ever since the middle of last century, lithium hydroxide began to
be added to the electrolyte of alkaline batteries. It was found that the addition of this substance to the
electrolyte alkaline battery increases the battery capacity approximately by 20% and makes the
service life 2 ÷ 3 times longer. In recent years, this area has dramatically expanded in connection with
the creation of lithium power sources required for the operation of cell phones and other small
consumer electronics devices.
Lithium is used in manufacturing anodes for chemical current sources of accumulator batteries
and battery cells with solid electrolyte working on the basis of non-aqueous solid and liquid
electrolytes. Examples of such batteries are lithium-chromium-silver accumulators. Electrochemical
cells with solid electrolytes are represented by the following designs: lithium-bismuth, lithium-copper
oxide, lithium dioxide-manganese, lithium-iodine-lead, lithium-thionyl-chloride, lithium-oxide
vanadium, lithium-fluorine-copper, and lithium-dioxide-sulfur elements. In case of liquid electrolytes
the following organic polar liquids are used as solvents: tetrahydrofuran, propylene carbonate, methyl
formate, acetonitrile, and others.
When used in lithium batteries as positive electrodes, such solid electrolytes as lithium cobaltate
and lithium nickelate showed the best performance and energy consumption characteristics. Currently,
the following several classes of cathode materials are used in mass production of lithium-ion batteries:
- lithium cobaltate LiCoO2 and solid solutions on the basis of isostructural nickelate LiNiO2;
- Lithium-manganese spinel LiMn2O4;
- Lithium-ferrophosphate LiFePO4.
- Carbon-coated nanotubes of lithium molybdate, which can be used as an anode in lithium-ion
accumulators [33].
Lithium aluminate (lithium β-alumina) is one of the most effective solid electrolytes, along with
sodium and cesium-β-alumina.
Lithium hydroxide is used as a component for preparation of an alkaline battery electrolyte. Due
to the formation of lithium nickelates an addition of lithium hydroxide to an electrolyte traction iron
nickel, nickel-cadmium, or nickel-zinc rechargeable battery increases the battery capacity by 12%, the
resistivity at 21%, extends the service life of nickel-iron battery 2 ÷ 3 times, and increases its capacity
by 21 %.
Almost the same area includes the use of lithium compounds as thermoelectric materials. Thus,
the alloy of lithium sulfide and copper sulfide is an effective semiconductor for thermoelectric
converters. It has the EMF about 530 µV/K.
Glass and Ceramics
Ceramics and glass are the second most important areas of application of lithium and its
compounds. Ceramics that are solidify at room temperature and used in military equipment,
metallurgy and, in the long term, in fusion energy is made on the basis of lithium aluminate and
lithium silicate. Glass based on lithium-aluminum-silicate reinforced with silicon carbide fibers has a
tremendous strength.
A significant number of lithium compounds are used to produce glass having such properties as
improved chemical resistance, transparency to ultraviolet or infrared radiation, and photosensitivity.
The introduction of lithium compounds contributes to the production of high-quality ceramics. In
particular, lithium is included in the high voltage porcelain composition, and the composition of
specific ceramic coatings having exceptional heat resistance (Stupalit) that are used to extend the life
of combustors and nozzles of jet engines. In enamels lithium compounds reduce viscosity of the melt
and facilitate formation of smooth and thin coatings. Lithium is a component of a high-quality glaze.
Lithium and its compounds are widely used in the silicate industry for the production of special
types of glass and for application of porcelain coatings.
Lithium oxide comprises a widely used flux for treatment of silicon dioxide by reducing the
melting point and viscosity of the molten material and thus improving physical properties of glaze
coatings, as well as reducing the thermal expansion coefficients. Lithium oxide is a component used in the manufacture of glassware. As a rule, lithium carbonate (Li2CO3) is used in the art since it is
converted into an oxide by heating.
Liquid lithium glass is among the most tonnage of lithium products. It is widely used in a variety
of silicate cements in construction industry. In metallurgy it is used as a binder for foundry forms.
When lithium silicates lose moisture at a temperature in the region of 150÷200 ℃, they begin to
convert into forms insoluble in water and quickly become waterproof. Lithium polysilicate solutions
are mainly used as a binder in zinc anticorrosion coatings, providing stability and storability of paints.
Furthermore, these solutions are suitable for depositing thin silica films on a variety of surfaces,
including glass surfaces of optical devices. Lithium hydroxide, as well as lithium silicate or
polysilicate are good additives to the sodium or potassium silicate systems modifying their properties.
Lubricants
The third most important area of application of lithium compounds includes lubricants. Lubricants
can be solid, plastic, liquid, or gaseous substances that are used in friction units of motor vehicles,
industrial machinery, as well as in everyday life for reducing wear of moving mechanisms and
structures caused by friction. Lubricants are presented by lubricating oils and greases. Greases are
substances that are in a pasty state at ordinary temperatures and in a liquid state when heated. They
are complex colloidal systems having a solid phase consisting of a thickener (sometimes filler) and a
liquid phase consisting of a mineral oil.
The most important properties of greases are consistency, i.e., the degree of density of grease;
melting point that characterizes the upper limit of the working temperature of the lubricant; chemical
and mechanical stability; colloidal stability, i.e., resistance against decay into liquid and solid phases;
and thermal stability, i.e., the ability to retain its structure and properties during prolonged heating.
Examples of such materials are Litol and CIATIM-201. They are mixtures of low-viscosity
petroleum oils thickened with lithium salts of fatty acids. One of the most common compounds of this
type is lithium stearate (“lithium soap”), which is used as a thickener for pasty high temperature
greases for machines and mechanisms. Lithium salts of higher fatty acids are the basis of most high
quality greases with an unusually wide operating temperature range (-60 to + 160°C).
Thickeners are important components for preparation of greases. Soaps may also be used as
thickeners for increasing viscosity of oils. The major components of most grease are usually
emulsions of mineral oils and calcium or lithium soaps. Widely used are calcium and lithium greases.
The lithium soaps are also used as thickeners for increasing the viscosity of oils.
Soaps are the most common emulsifying agents, and a choice of a specific type of soap depends
on its specific application. The soaps typically comprise calcium, sodium and lithium stearates or
mixtures of these components. Frequently used are soaps based on fatty acids which differs from
stearate derivatives. For example, particularly widely used is lithium 12-hydroxystearate. The lithium
12-hydroxystearate exhibits high stability to oxidation approximately to about 200°C. Most lubricants
used today in cars, airplanes and heavy equipment include stearates of lithium, mainly lithium 12
hydroxystearate [34]. Greases may be prepared with the addition of several different metallic soaps.
Lubricants based on lithium soaps are preferred because of their resistance to water, as well as their
mechanical and oxidative stability. They also have good characteristics, both at high and at low
temperatures. Greases based on lithium have a higher melting temperature than calcium-based greases.
Lithium lubricants have melting points in the range of 190 to 220 ℃. However, the maximum usable
temperature for lithium grease is 120 ℃.
Being a strong base, lithium hydroxide, when heated with fat, forms a soap containing lithium
(lithium stearate). Lithium soap has the ability to thicken oil, and it is used for the production of
universal, high-temperature greases [35].
Regeneration of oxygen in the autonomous life support systems
In its importance this area takes the fourth place among the fields in which lithium compounds are
used and is one of the most underdeveloped applications of lithium. Lithium hydroxide LiOH and
lithium peroxide Li2O2 are used to clean the air from carbon dioxide and, when the latter compound
reacts, it evolves oxygen
2𝐿𝐿2𝐶2 + 2𝐶𝐶2 → 2𝐿𝐿2𝐶𝐶3 + 𝐶2 ↑
Due to this effect, it is used in insulating gas masks, cartridges for purification of air in
submarines, manned spacecraft, etc.
Anhydrous lithium bromide LiBr and lithium chloride LiCl, which possess high hygroscopicity,
are used to maintain constant humidity by drying air and other gases.
Polymer materials and chemical technology
Fifth place in the structure of the lithium consumption takes lithium organic synthesis and
especially production of polymeric materials and in particular synthetic rubber. The basic lithium
compound used in these processes is butyllithium (BuLi). Organic lithium compounds and, in
particular butyl lithium, are obtained from metallic lithium and alkyl halides [32]. The main area of
application of butyllithium for industry is use thereof as a catalyst for anionic polymerization in the
manufacture of polyisoprene, polybutadiene and butadiene/styrene, which play an important role in
the production of plastics and rubber. Thus butyllithium is used as an initiator of anionic
polymerization of dienes such as butadiene. This reaction is called a reaction of carbonylation:
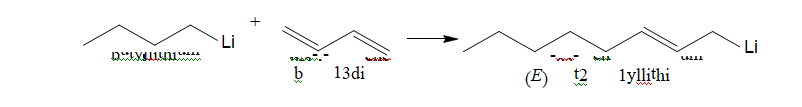
Thus, stereospecific polymerization of isoprene can occur. Also commercially important is the use
for the manufacture of butyllithium styrene-butadiene polymers. Even an ethylene can be polymerized
under the influence of BuLi.
By using butyllithium it becomes possible to control a homogeneous polymerization for obtaining
polymeric material having predetermined molecular weights, molecular weight distributions, given
ratios of co-monomers, sequences of butadiene and styrene monomer units, and degrees of branching.
Thus, it is possible to produce the most effective microstructure [29-31].
In addition, butyl lithium, which is a valuable tool in organic synthesis of active pharmaceutical
ingredients (API), is used in the production of chemicals for agriculture and electronic materials. Due
to distinct properties of its base, the butyl lithium can be used as a universal reagent for metallization
(deprotonation or metal-halogen exchange) of organic matters.
n-BuLi belongs to a group of compounds called “superbases” and is one of the strongest bases
which are used in organic synthesis. When dissociation constant (pKa > 35) is set as an acid, the n
butyllithium is capable of deprotonating a wide range of acidic compounds thus allowing further
conversions, such as generation of C-C bonds. Mild reaction conditions, high yield, an insignificant
amount of byproducts, and ease in detaching residual lithium salts are the main advantages of n
butyllithium in comparison with alternative reagents. For specific reactions of deprotonation can be
used sec-butyl lithium or t-butyl lithium having even more pronounced basic properties.
Another common use of butyl lithium as a reagent in organic synthesis is use thereof in metal
halide exchange reactions. In this type of reactions, aliphatic or aromatic organic halides (generally
iodides or bromides) are employed as substrates. A reaction with butyllithium generates fewer
organolithium compounds, and this increases the scope for further intermediate reactions with suitable electrophilic agents. Compared with other classical methods such as the use of magnesium-organic
compounds, the halogen exchange with butyl lithium is characterized by mild reaction conditions,
high yields, and a small amount of by-products, especially when using compounds with functional
groups sensitive to harsh conditions.
Many other lithium compounds are used as reagents for the preparation of organic compounds.
Examples of such compounds are lithium aluminum hydride (LiAlH4), lithium borohydride (LiBH4),
lithium triethyl borohydride (LiBH(C2H5)3), etc.
Metallurgy
Ferrous and non-ferrous metals are also one of the great applications for metallic lithium, which is
used for deoxidation and improving the ductility and toughness of alloys. Lithium is administered as a
single metal or with metals of different ligatures (e.g., 30% Li + 70% Ca) for deoxidizing, alloying, or
modifying many brands of ferrous alloys and irons. It is used to increase the yield, strength, and
hardness of carbon steel and stainless steel, for modifying the high-speed tool steel and austenite steel.
In non-ferrous metallurgy, the introduction of 1% lithium into magnesium alloy improves its corrosion
resistance and resistance to tearing. Of great interest is the ability to create “floating” Mg-Li alloys
containing more than 50% Li.
By using metallothermic methods, lithium is sometimes employed for recovery of rare metals.
Metallothermy is recovery of metals at elevated temperatures from their compounds with other metals
which are chemically much more active than the recoverable.
Special application of lithium in metallurgy is metallurgy of aluminum. Thus, lithium carbonate is
an essential adjunct (for addition to electrolytes) in the smelting of aluminum, and every year its
consumption grows in proportion to the volume of world aluminum production (consumption of
lithium carbonate is 2,5 ÷ 3,5 kg per ton of smelted aluminum).
Lithium is also widely used for aluminum alloying. Introduction of lithium into the doping system
makes it possible to obtain new aluminum alloys of high specific strength. Addition of lithium
reduces the density of the alloy and increases its modulus of elasticity. However, high concentrations
of lithium in aluminum alloys may affect their corrosive properties. At lithium content up to 1.8% the
alloy becomes low resistant to stress corrosion and at 1.9% becomes not prone to stress corrosion
cracking. The increase in lithium content up to 2.3% contributes to increase in the probability of
formation of cracks and loose structures. This changes the mechanical properties of aluminum-lithium
alloys. In other words, increase in limits of their strength and yield is accompanied by decrease in
plastic properties of such alloys.
The most famous doping system are Al-Mg-Li (an example is alloy 1420 used in the manufacture
of aircrafts) and Al-Cu-Li (an example is alloy 1460 used in the manufacture of tanks for liquefied
gases). An alloy on the basis of aluminum, which is known as “Skleron” and which contains lithium,
has found use in the aircraft industry. Tensile strength, elasticity and hardness of this alloy are higher
than the same properties in duralumin-type alloys. Alloys of lithium with magnesium, scandium,
copper, cadmium, and aluminum are new promising materials in aviation and astronautics (because of
their lightness). Excellent mechanical properties are in lithium alloys with copper and lead. Lithium is
very effectively strengthens lead alloys and gives them flexibility and resistance to corrosion. Alloys
of lithium with titanium, beryllium, zinc, and silver also find application. Alloys of lithium with silver
and gold, as well as with copper, are very effective solders.
Lithium is sometimes used for recovery of rare metals from their halides by metallothermic
methods.
Pharmaceuticals, medicine and biological importance of lithium
Lithium salts exhibit normothymic and other therapeutic properties. Therefore, they are used in
medicine.
Lithium drugs are psychotropic medical substances from normothymics. Historically they are first
drugs of this group, found yet in 1949; however, they remain essential in the treatment of affective
disorders, especially manic and hypomanic phases of bipolar disorders, as well as in their preventive
treatment and treatment of exacerbations of severe or resistant depressions, as they possess preventive
properties. Lithium preparations have some other applications as well [36,37].
Lithium is an alkaline metal, so it is used in medicine in the form of salts, mostly as carbonates
and also as citrates, succinates, orotates, chlorides, and sulphate of lithium. Lithium bromide is no
longer used in medicine, as it causes chronic poisoning – bromism – already at a dose of 250 mg per
day. In Russia, only carbonates are used now from the salts of lithium, and hydroxybutyrate and
lithium nicotinate were used previously.
Human body needs lithium in small quantities (about 100 mg/day for adults). Lithium is present in
the human body predominantly in the thyroid, lymph node, heart, liver, lungs, intestine, blood plasma,
and adrenal glands.
Lithium is involved in important processes:
- participates in carbohydrate and fat metabolism;
- supports the immune system;
- prevents the occurrence of allergies;
- decreases nervous excitability.
Lithium is evolved mainly by kidneys.
Some lithium compounds are used as catalysts in medicine for drug development. Lithium soaps
are used for impregnation of water-repellent fabrics.
Other areas which are close by use of lithium to pharmaceutics are the textile industry where
lithium compounds are used for bleaching fabrics, and the food industry where lithium compounds are
used for food preservation. Lithium compounds are also used in the manufacture of cosmetics.
Prospective application field of lithium and its compounds
There are a variety of other applications for lithium compounds. Many of them are historically
developed, and some are new as they arise from the development of science and technology in recent
years. These applications are also in the near future may be at the forefront of lithium consumption
and its compounds. It is therefore necessary to carry out intensive research in these areas, as the most
promising.
Hydrogen energetics
The main problem of hydrogen energetics is the problem of hydrogen storage. Methods of storing
hydrogen for later use cover a variety of approaches, including high-pressure, cryogenic techniques,
and chemical compounds that reversibly release H2 under certain influences. Most researches on
hydrogen storage are focused on storing hydrogen in the form of solid compounds – hydrides as light
and compact energy sources for mobile applications. Comparison of different methods of hydrogen
storage is presented in Figure 2.
Metal hydrides are represented by squares and complex hydrides (including LiAlH4) by triangles.
BaReH9 has the highest known ratio of metal and hydrogen (4.5), Mg2FeH6 has the highest known
bulk density of H2, LiBH4 has the highest weight density. The values presented for the hydrides
exclude the role of capacity. Objectives are DOE including the weight of the container [40].
Lithium hydride LiH has the higher hydrogen content out of other hydrides, and more than three
times exceeds content of hydrogen in sodium hydride NaH. The LiH is of great interest for hydrogen
storage, but its resistance to decomposition is an obstacle to practical use. Thus, removal of H2
requires temperatures above 700°C needed for its synthesis, and such temperatures are costly to build
and maintain.
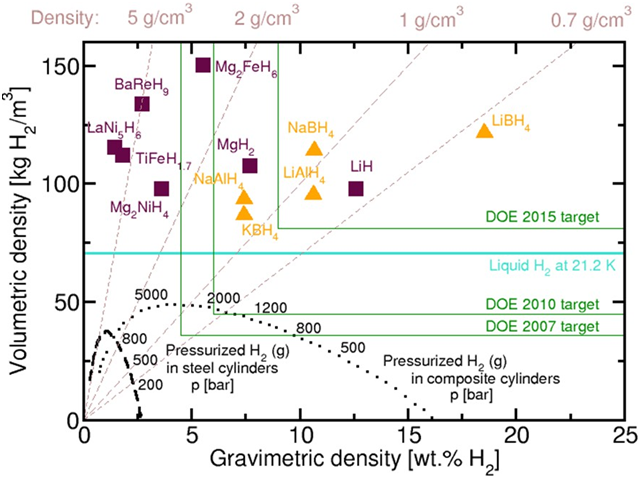
Figure 2. The volume and weight densities of hydrogen at different methods of storage
Metal hydrides such as MgH2, NaAlH4, LiAlH4, LiH, LaNi5H6, TiFeH2 and palladium hydride
which were used with varying degrees of success, also may be employed as hydrogen storages, often
reversible [38]. These materials have a good energy density in volume, although their weight energy
density is often worse than that of the basic hydrocarbon fuels. Most metal hydrides have very strong
bind to hydrogen. As a result, the hydrogen contained therein is released at high temperatures of about
120 ÷ 200 ℃. The energy costs can be reduced by using mixed compounds, such as in LiNH2, LiAlH4
and NaBH4 [39].
LiAlH4 contains 10.6% by weight of hydrogen, which makes it a potential hydrogen storage
medium for future use in vehicle fuel cells. The high content of hydrogen, as well as finding of the
reversible hydrogen storage properties in NaAlH4 doped with Ti [36] called over the last decade for
the resumption of LiAlH4 research. Significant research efforts have been directed to acceleration in
decomposition kinetics by catalytic doping and grinding in a ball mill [37]. In order to take advantage
of the total capacity of the hydrogen, the intermediate LiH must be dehydrogenated as much as
possible. Because of high thermodynamic stability of the product of hydrogenation, the temperatures
should exceed 400°C, which is not considered feasible for transport purposes. Receiving LiH + Al as
a final product reduces the hydrogen storage capacity to 7.96 wt. %. Another problem associated with
hydrogen storage is inverse recirculation of LiAlH4, which, due to its relatively low stability, requires
extremely high hydrogen pressure in excess of 10,000 bar [37].
Lithium borohydride NaBH4 is known as one of chemical energy carriers that has the highest
energy density. The high specific energy density of lithium borohydride made it an attractive
candidate that can be offered for automotive and rocket fuel, but, in spite of the research and advocacy,
it is not yet widely used. Like all energy-based hydrides, lithium borohydride is very complex for
utilization (i.e., recharge) and therefore suffers from low efficiency of energy conversion. At a density
of 0.67 g/cm3, solid lithium borohydride releases 65 MJ/kg of heat by reaction with oxygen. For
comparison, gasoline gives 44 MJ/kg, whereas the liquid hydrogen provides 120 MJ/kg. Thus lithium
ion batteries have power density up to 0.72 MJ/kg, but the efficiency of energy conversion can reach
90%. In view of the complexity in the mechanisms of cyclic use of metal hydrides, such high energy
conversion efficiency is beyond the practical reach. However, due to the high energy consumption,
their use will probably be in the region schemes of compromise.
It this well-known that in a reaction of lithium hydride with water:
𝐿𝐿 + 𝐿2𝐶 → 𝐿𝐿𝐶𝐿 + 𝐿2↑
1 kg of lithium hydride may evolve 2.8 m3 hydrogen. Therefore hydride for many years is used as
a solid source of hydrogen to fill the survival crafts, balloons, etc. Thus, lithium hydride and lithium
aluminum hydride can be easily used to generate hydrogen by reacting them with water. It is hoped
that such their use will have great prospects for hydrogen energetics.
Electronics and nonlinear optics
Mixed cesium lithium borate is used as an optical material in electronics. Single crystals were
obtained from triborate lithium – LiV3O5 (LBO) and double-cesium lithium borate – CsLiB6O10
(CLBO). These single crystals, having a relatively high non-linear optical properties, a wide area of
transparency and high radiation resistance, quickly found a wide application in laser instruments [17].
The crystalline lithium niobate LiNbO3 and lithium tantalate LiTaO3 are non-linear optical
materials which are widely used in nonlinear optics, acoustic optics, and optoelectronics. In particular,
they are used in electro-optical modulators, pyroelectric detectors, and piezoelectric transducers.
Lithium is also used in filling gas-discharge metal halide lamps.
Single crystals of lithium fluoride LiF are used for the manufacture of high-performance (80%
efficiency) lasers in free color centers and for the manufacture of optics with a broad spectral
bandwidth.
Lithium sulfate is used for the manufacture of detectors in ultrasonic defectoscopy.
Nuclear energy
The isotopes 6Li and 7Li have different nuclear properties – cross section of thermal neutron
absorption and scope of their application are different. Lithium hafniate is a part of special enamel
intended for the disposal of high level nuclear waste that contains plutonium.
Lithium-6 is used in nuclear energetics.
An important area of nuclear applications of lithium is the use of hydride 6LiH in light shields
against neutron radiation.
Irradiation of 6Li nuclide with thermal neutrons produces radioactive tritium 3H:
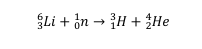
Therefore, lithium-6 can be used as a replacement for tritium, which is radioactive, unstable, and
inconvenient in handling. Such a replacement takes place both for military purposes in the production
of thermonuclear weapons and for peaceful purposes in control of thermonuclear fusion. In
thermonuclear weapons it is generally used in the form of lithium deuteride – 6LiD.
Promising also is the use of lithium-6 for the production of helium-3 through tritium for the
purpose of further use in deuterium-helium fusion reactors.
Creation of an economic and safe fusion reactor requires a development of special structural
materials for the first wall, blanket, breeders and for elements working in the conditions of high heat
fluxes. Materials must be chemically compatible with the coolant and tritium reproducing materials,
such as lithium. The breeder’s function is an effective, safe and reliable production of tritium from
lithium under neutron irradiation. Since the heat generated by a nuclear reaction is absorbed by the
blanket and is transferred to the coolant, it is important to know the structure and physical-mechanical
properties of the breeder material and the breeder and changes occurring in this material during
burning lithium out from this material.
Currently, the following basic materials are planned to be used in ITER reactor (International
Thermonuclear Experimental Reactor):
- low-activated steels, vanadium alloys, and ceramics based on lithium for solid blankets of
Li2TiO3, Li2SiO4, etc.; and - eutectic based on lithium for the blanket of the eutectic alloy Li-Pb [41].
Oxide ceramics based on lithium are considered as materials perspective for solid breeder
blankets in future fusion reactors. It is believed that one of the materials is basic perspective breeder
material such as lithium methatitanate Li2TiO3 which lately attracts most attention because of the
high rate of release of tritium at relatively low temperatures (from 200 to 400℃) and chemical
stability.
Studies over the prospects for the use of lithium in ceramics in the tritium breeder of installation
of controllable thermal nuclear units are held in Europe, Japan, and Russia [42].
Lithium-7 is used in nuclear reactors which have a reaction involving heavy elements such as
uranium, thorium, or plutonium.
Due to a very high specific heat and low thermal neutron capture cross-section, liquid lithium-7,
often serves as an effective coolant in the form of an alloy with sodium or cesium. Lithium-7 fluoride
7 in an alloy with beryllium fluoride (66% LiF + 34% BeF2) is called «flibe» (FLiBe) and is used as a
highly efficient heat transfer fluid and solvent for fluorides of uranium and thorium in high
temperature molten-salt reactors and in production of tritium.
Liquid lithium-7 can be used as a coolant in a nuclear reactor due to its large temperature range in
the liquid state (179 ÷ 1317 °C), low density (ρ = 0,534 g/cm3), high heat capacity, relatively low
viscosity, high heat of vaporization, and a small thermal neutron capture cross section (up to 16 times
less than that of sodium). An obstacle to the use of liquid lithium is its strong corrosive action.
Lithium-7 deuteride may serve as a more effective neutron moderator, better than hard water.
The compounds of lithium enriched in the isotope of lithium-7, is used in PWR reactors to
maintain water-chemistry regime, as well as in the desalter of the first reactor cooling circuit. Annual
demand of the United States is estimated as 200-300 kg. This product is manufactured only in Russia
and China [11].
Rocket fuel
According to foreign experts, a possible field of application of lithium compounds is missiles
where the propellant may be comprised of lithium hydride, lithium boride and metallic lithium.
Combustion of 1 kg of lithium or some of its compounds gives up to 4000 kcal (conventional rocket
fuel – kerosene – 2300 kcal/kg). Perchlorate and lithium nitrate are characterized by a high oxygen
content (60.1 and 69.5%, respectively), and may be used as an oxidant, while ammonium perchlorate
which is used in the solid rocket fuel as an oxidizer contains only 54.4% oxygen.
Metallic lithium has been tested as a fuel in rocket engines. The results of these tests with various
oxidants gave positive results which are presented in Table 3.
Lithium hydride LiH and lithium aluminum hydride LiAlH4 were tested as solid propellants for
some models of rockets [28]. In addition, lithium nitrate is a classic product that is used in
pyrotechnics for coloring in red lights.
Other applications
A very promising use of lithium is filler for a bathyscaphe float because this metal has a density
that is almost two times lower than water (more specifically, 534 kg/m³). This means that one cubic
meter of lithium can keep afloat almost by 170 kg more than one cubic meter of gas. However,
lithium is an alkali metal and therefore actively reacts with water. Therefore it should be reliably
isolated from water in order to prevent their contact. A saturated solution of LiCl is used as a de-ice because it has a very low freezing point -55 ℃.
Table 3. Theoretical characteristics of rocket fuels formed by lithium with various oxidants
| Oxidizer | Specific pulse(Р1,с) | Combustion temperature,°С | Fuel density, g/cm³ | Masscontent of fuel, % |
| Fluorine | 378.3 | 5350 | 0.999 | 28 |
| Tetra-fluoro- hydrazine | 348.9 | 5021 | 0.920 | 21.07 |
| ClF3 | 320.1 | 4792 | 1.163 | 24 |
| ClF5 | 334 | 4946 | 1.128 | 24.2 |
| Perchlorofluoride | 262.9 | 3594 | 0.895 | 41 |
| Oxygenfluoride | 339.8 | 4595 | 1.097 | 21 |
| Oxygen | 247.1 | 3029 | 0.688 | 58 |
| Hydrogen peroxide | 270.5 | 2995 | 0.966 | 28.98 |
| N2O4 | 239.7 | 3006 | 0.795 | 48 |
| Nitric acid | 240.2 | 3298 | 0.853 | 42 |
COST OF LITHIUM
The price of lithium has not been widely published. By various sources, on the average for the
period of 2007- 2008, the price of metallic lithium (99% purity) was $63-66 per 1 kg. By August 2009,
it did not change and amounted to about US $6,600 per ton. These prices can be compared with the
price at the beginning of the last decade, when it was about $ 2,500 per ton.
Currently Public Broadcasting states that “the cost of lithium is an eightfold increase in the last six
years”. It is also predicted that the price of lithium will continue to actively grow with increasing
demand for energy sources containing lithium.
After the financial crisis of 2007, the cost of lithium from major suppliers such as Sociedad
Química y Minera (SQM) dropped by 20% [43]. Prices rose in 2012. In 2012, an article in Business
Week outlined the oligopoly in the area of lithium – a market form, which is dominated by a small
number of sellers. Thus SQM controlled by billionaire Julio Ponce, is the second largest, following
Rockwood Lithium Inc., which operates with the support of KKR & Co. of Henry Kravis and of FMC
which is based in Philadelphia. To meet the demand for lithium batteries, which is growing at 25
percent per year, the global lithium consumption may rise from about 150 000 tons of 2012 to
300,000 metric tons per year by 2020. Demand growth is ahead by 4 ÷ 5% of the total growth of
lithium production [44].
DEMAND FOR LITHIUM
Lithium market has steadily grown over the past decade due to the increasing use of lithium
batteries in consumer electronics, such as cell phones, laptop computers, and other portable electronic
devices. Known devices such as IPods and Blackberries, quickly turn to lithium as a component of the
main sources of energy, as such sources are comfortable, light in weight, and reliable in operation.
But excitement among many investors results mostly from the expected models of electric cars
that may be created by Chevy Volt, Nissan Leaf, and Mitsubishi. Some automobile companies are all
speculating on the fact that pure electric cars could account only for 10% of purchases of cars by 2020.
Despite this, the automobile giant such General Motor tries to get a large piece of the lithium pie.
Companies such as Apple, Motorola, Research in Motion, Hewlett-Packard, Dell, Samsung and
Sony all use lithium in majority of their products.
REFERENCES
- Pilson, M.E.Q. An introduction to the chemistry of the sea / M.E.Q. Pilson, University of
Rhode Island. – Second edition. Cambridge university press, NY, 2013, 543 p. - Jaskula B.W. Minerals Yearbook – 2008, Lithium, U.S. Department of the Interior, U.S.
Geological Survey, October 2010. - Lithium. Statistics and Information.USGS Mineral Commodity. http://minerals.usgs.gov/
minerals/pubs/commodity/lithium/ - Bolivia’s Lithium-Powered Future. http://foreignpolicy.com/slideshow/bolivias-lithium
powered-future/ - Evans R.K. An Abundance of Lithium, March 2008, http://www.che.ncsu.edu/ILEET/
phevs/lithium-availability/An_Abundance_of_Lithium.pdf - Burns E. Pay Dirt http://evworld.com/library/pay_dirt.pdf
- Managing Critical Isotopes. Stewardship of Lithium-7 Is Needed to Ensure a Stable Supply.
GAO-13-716, September 2013. - PWR – литиеваяугроза. http://www.atominfo.ru/newsf/m0910.htm
- http://www.rockwoodlithium.com/
- http://www.lithiumcorporation.com/index.php
- Managing critical isotopes. Stewardship of Lithium-7 Is Needed to Ensure a Stable Supply.
Report to the Ranking Member, Subcommittee on Oversight, Committee on Science, Space,
and Technology, House of Representatives, US Government Accountability Office, GAO-13
716, September 2013. - Onorin S.A., Volkhin V.V., Method for producing the inorganic ion exchangers, USSR
Author’s Certificate № 455560, 1972 - Volkhin V.V., Leontieva G.V., Cheraneva L.G., Bakhireva O.I., Lithium desorption method
with an inorganic ion exchanger on the basis of manganese and aluminum oxides, RF patent
№1811679, 1991. - Kudryavtsev P.G., Onorin S.A., Volkhin V.V. The process for producing inorganic sorbent
for extracting lithium from solutions, USSR Author’s Certificate № 1160627, 1983 - Kudryavtsev P.G., Onorin S.A., Volkhin V.V., Yakimov V.A. The process for producing
inorganic sorbent selective to lithium, USSR Author’s Certificate № 1256274, 1985 - Onorin S.A., Volkhin V.V. Composition for inorganic ion exchanger, USSR Author’s
Certificate № 451 456, 1972 - Yurkin A.M. Growth and properties of single crystals of lithium borates, lithium, cesium and
barium, dis. Ph.D., 01.04.14, Novosibirsk, 2002, 161 p. - Garret D.E., Laborde M. Recovering lithium from brine by salting out lithium sulfate
monohydrate. Pat. USA N 4287163, 01.09.1981. - Berzain R.L. Method for concentration of lithium chloride from Salarde Uyuni brines. Rev.
Boliv. Quim., 1985, v. 5, N 1, p. 8—20. In CA, 1986, v. 104, ref. N 227124 h. - Lee J.М., Bauman W.C. Recovery of magnesium (2+) from brines. — Pat. Can. N 1103399,
16.09.1981. In CA, 1982, v. 96, ref. N 38797 k. - Zil’berman M.V., Kalinin N.F., Chentsova T.V., Yelizarova I.A., Antipov M.A., Murav’yev
Ye.N. Sorption technology for processing natural brine in lithium compound, rubidium and
cesium, Chemistry and technology of inorganic sorbents. Interuniversity collection of
scientific papers. Perm, ed. Perm PI, 1989, s.5-9. - Senyavin MM Krachak AN Nikashina VA Study of lithium adsorption of highly mineralized
solutions on different types of inorganic ion exchangers, Chemistry and Technology of
Inorganic sorbents, Interuniversity collection of scientific papers, Perm, ed. Perm PI, 1989,
s.10-26. - Bengtsson G.B., BortunA.I., Strelko V.V. Strontium Binding Properties of Inorganic Adsorbents, Journal of Radioanalytical and Nuclear Chemistry, Articles, Vol. 204, No. 1, 1996, p.75-82
- Belenitskaya G.A. Tectonic aspects of the spatial and temporal distribution of salt basins in
the world, electronic scientific publication Almanac Space and Time. Vol. 4, Issue 1, 2013,
Special Issue: Earth system, s.1-31. - Israel useful resources (Wikipedia, the freeencyclopedia) https://ru.wikipedia.org/wiki/%D0%9F%D0%BE%D0%BB%D0%B5%D0%B
7%D0%BD%D1%8B%D0%B5_%D0%B8%D1%81%D0%BA%D0%BE%D0%BF%D0%B
0%D0%B5%D0%BC%D1%8B%D0%B5_%D0%98%D0%B7%D1%80%D0%B0%D0%B8
%D0%BB%D1%8F - Nissenbaum, A. Trace Elements in Dead Sea Sediments. Israel Journal of Earth Science, 1974,
Vol. 23, p. 111-116. - Nissenbaum A. Minor and trace elements in Dead Sea water, Chemical Geology, Volume 19,
Issues 1–4, 1977, P. 99-111 - Wietelmann U., Bauer R.J. “Lithium and Lithium Compounds” in Ullmann’s Encyclopedia of
Industrial Chemistry, 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a15_393 - Yurkovetskii, A.V.; Kofman, V.L.; Makovetskii, K.L. (2005). Polymerization of 1,2
dimethylenecyclobutane by organolithium initiators. Russian Chemical Bulletin 37 (9): 1782 - doi:10.1007/BF00962487.
- Quirk, R.P.; Cheng, P.L. (1986). Functionalization of polymeric organolithium compounds.
Amination of poly(styryl)lithium. Macromolecules 19 (5): 1291–1294. doi:10.1021/
ma00159a001. - Stone, F.G.A.; West, Robert (1980). Advances in organometallic chemistry.Academic Press.p.
55.ISBN 0-12-031118-6. - Bansal, Raj K. (1996). Synthetic approaches in organic chemistry. p. 192.ISBN 0-7637-0665
- Liu Xudong, LyuYingchun, Zhang Zhihua, Li Hong, Hu Yong-Sheng, Wang Zhaoxiang,
Zhao Yanming, KuangQuan, Dong Youzhong, Liang Zhiyong, Fan Qinghua, Chen Liquan
Nanotube Li2MoO4: a novel and high-capacity material as a lithium-ion battery anode //
Nanoscale. 2014. Vol. 6. P. 13660-13667. DOI:10.1039/C4NR04226C
- Liu Xudong, LyuYingchun, Zhang Zhihua, Li Hong, Hu Yong-Sheng, Wang Zhaoxiang,
- Wietelmann, U.; Bauer, R.J. (2000). “Lithium and Lithium Compounds”. Ullmann’s
Encyclopedia of Industrial Chemistry. Wiley-VCH. - Totten, George E.; Westbrook, Steven R. & Shah, Rajesh J. (2003). Fuels and lubricants
handbook: technology, properties, performance, and testing 1. ASTM International.p.
559.ISBN 0-8031-2096-6. - Geddes J. R., Burgess S., Hawton K. et al. (2004). «Long-term lithium therapy for bipolar
disorder: Systematic review and meta-analysis of randomized controlled trials». The
American Journal of Psychiatry 161 (2): 217–222. PMID 14754766 - Bauer, Michael MD, PhD; Döpfmer, Susanne MD† (Oct 1999). «Lithium Augmentation in
Treatment-Resistant Depression: Meta-Analysis of Placebo-Controlled Studies». Journal of
Clinical Psychopharmacology 18 (5). - DOE Metal hydrides. 2015 Annual Progress Report. https://www.hydrogen.energy.gov/
annual_progress15_storage.html - Meganne L. Christian and Kondo-François Aguey-Zinsou. Core–Shell Strategy Leading to
High Reversible Hydrogen Storage Capacity for NaBH4, ACS Nano, 2012, 6 (9), pp 7739
7751, DOI: 10.1021/nn3030018 - https://en.wikipedia.org/wiki/Lithium_aluminium_hydride
- Tazhibayeva I., Beckman I., Shestakov V., Kulsartov T., Chikhray E., Kenzhin E.,
Kiykabaeva A., Kawamura H., Tsuchiya K. Tritium accumulation and release from Li2TiO3
during long-term irradiation in the WWR-K reactor, Journal of Nuclear Materials, Vol. 417,
2011,pp. 748–752 - Tsuchiya K., Nakamichi M., Nagao Y., Fujita J, Sagawa H., Tanaka S., Kawamura H.
Integrated experiment of blanket in-pile mockup with Li2TiO3 pebbles. // Fusion Engineering
and Design (Japan), v. 51-52, 2000, p.887-892 - http://www.prnewswire.com/news-releases/sqm-announces-new-lithium-prices
62933122.html - http://www.bloomberg.com/news/articles/2014-07-15/lithium-boom-drives-albemarle-6-2
billion-rockwood-deal







